Important Note: This is a draft version. This document is still currently in progress and will evolve in the coming months.
Today, millions of people around the world lack access to life-saving medicines because of high prices or lack of innovation. Health providers are in crisis, and have to make tough choices about what drugs they can afford to provide.
The source of the problem lies in how we reward medical innovation: by providing monopolies in the form of patents. Patents create an unavoidable conflict by using a single, per treatment, payment that must cover the cost of R&D at the same time as manufacturing costs. Under the patent system, innovators need high prices per pill to get paid and these high prices restrict the number of patients that can be treated.
Patents make this conflict between access and innovation inevitable but we could choose to to separate payment for R&D from payment for manufacture. This separation would remove the conflict and deliver strong incentives for innovators at the same time as greatly expanding access to medicines.
In this paper we explore a new model where we reward innovations using remuneration rights, and, in return, innovators provide unrestricted, royalty free access to their innovations for both manufacturing and research purposes. This would allow free-market competition in manufacturing, leading to prices close to cost of manufacturing, as well as faster, freer and more innovative research.
Is there a need for change?
The trajectory of the current system is unsustainable. Millions cannot get the treatment they need because of high prices, and innovation is inefficient; important disease areas are neglected, research is often slowed by legal disputes and licensing restrictions and a disproportionate amount of resources are used on areas of limited health impact. The patent system inevitably produces these tensions, as high prices for drugs are necessary to fund research and development.
Is there an alternative?
The remuneration rights model offers an alternative to the patent system. A remuneration rights fund would disburse payments to registered innovators based on health impact. In return, innovators would allow open access to all of their information, enabling generic competition in manufacture similar to out of patent medicines today. This would lower the prices of medicines without jeopardizing the funding of future research and development.
Under the remuneration rights system, taxpayers, employers and insurers would contribute towards healthcare payments just as they do today. Governments would pool these payments into an independant fund. Innovators would receive a remuneration right entitling them to a payment from the fund that reflects the health impact of their innovation. Patients would receive medicines in the same way as today, but at prices close to the cost of manufacture.
What is the impact on stakeholders?
Many groups hold a stake in how pharmaceutical research is financed. For some groups, such as politicians, shareholders, investors and basic researchers, the introduction of a remuneration rights system would make only limited changes. For others, such as patients, healthcare professionals, healthcare buyers, employers, insurance companies, taxpayers, marketers, distributors, manufacturers and innovators, the remuneration rights system would expand processes which already occur. There are also groups who would experience new roles as a result of the remuneration rights system, such as civil servants.
Are remuneration rights feasible?
Remuneration rights are technically feasible, and much of the relevant infrastructure is already in place; we already determine who own innovations and how rights can be shared between multiple innovators, and we already measure health impact. Remuneration rights are also politically feasible; sustainable funding can be ensured, governance established, and international cooperation achieved. In many instances, the relevant precedents are already in place under current systems, and national and international laws already permit remuneration rights. Moreover, transitions of this scale or larger have been successfully implemented in the past.
Such a transition can begin locally limited to a particular country or region and/or specific type of disease. Importantly it can operate in parallel with the patent system. A starting point would be a detailed feasibility study, followed by a pilot implementation of remuneration rights on a limited basis.
Are remuneration rights an improvement on patents?
The key criteria for assessing improvement would be societal welfare, equating to maximizing the number of healthy life years over the long-term. Roughly, this boils down to access, innovation and cost: it being desirable to have more access,1 more innovation and lower cost.
Remuneration rights provide increased access compared to patents. Generic competition would lower drug prices, while tying remuneration to health impact would increase the number of drugs available to poorer populations and incentivize distribution.
Remuneration rights offer the same or increased levels of innovation. Remuneration rights offer the same level and type of financial incentives as patents. Innovation might also become more effective by remunerating according to health impact rather than sale volume and allowing open access to information.
Finally the cost of the remuneration rights system for medicines would be the same or lower than our current patent-based system. The cost of the system is made of three parts: paying for innovation, paying for manufacture and paying for administration (e.g. the patent office etc). By far the largest of these is the expenditure on innovation and manufacture of medicines. Under remuneration rights these would be at a similar level to today. Administration costs would also be similar to today as remuneration rights reuse existing systems for granting and administering rights.
Pharmaceutical research and development has delivered some of the greatest achievements of the modern age, all but eradicating scourges that have terrorized humanity for generations and improving the health and quality of life of billions. But it is increasingly clear that the system by which we finance this progress is broken. Around the world millions lack access to the medicines they need to survive and thrive. Often, drugs are just too expensive, while in many areas of urgent need there are simply no medicines available to help.
The problem is global. In less affluent countries, where many pay out of pocket for medication, prices for even a short course of treatment can run to many times the average annual salary. In more developed nations, public healthcare systems struggle to afford soaring drug prices, having to ration or restrict access to the latest treatments.
These failures in access are a natural consequence of how we fund and incentivize medical innovation. Today, innovators are awarded a monopoly right on their inventions, a patent, which affords them exclusive marketing rights over the resulting product. As the research and development of new drugs is an extraordinarily expensive endeavor, the final prices innovators set on drugs are often very high in order to recoup costs, even though the actual drug itself may be relatively cheap to produce. As innovators are only paid through the final sale of the drug, prices must be high to cover these research costs. Necessarily, these high prices limit the number of people able to access the drug.
This system also skews what kinds of innovation we receive. Medicines which sell are incentivized rather than those with the most beneficial impacts on health. The closing off of vital research and information also slows down research progress. Therapies targeting chronic conditions for the wealthy have far greater potential for profit than those for acute conditions and for the global poor, and are invested in accordingly. Anti-aging products of limited health impact receive much more funding than debilitating and life-threatening conditions that impact billions.
Under the patent system - where innovators are only rewarded through the final sale of their product - access and innovation necessarily conflict; if we wish to develop any new medicines tomorrow then we must, reluctantly, restrict access today.
It is tempting, especially with increasingly strict protections on intellectual property rights worldwide, to view this scenario as an unfortunate inevitability. But there are ways to resolve the tension between access and innovation. Separating the funding of research and development from the sale prices of drugs would enable both access and innovation to flourish. We urgently need to seize this opportunity if we are to avoid the collapse of pharmaceutical innovation and healthcare as we know it.
The patent system, fitting in with a wider culture of intellectual property protection worldwide, has been so successful and dominant that it is easy to forget the instances where medical innovation has thrived without such protections. Opening up the products of research and development for wider use does not necessarily entail unsustainability and first copies going unpaid for. Even today, approximately half of all medical R&D in the United States is funded directly by the government. The histories of pharmaceutical development in India, Argentina and Italy, which did not always afford patent protection, illustrate that private sector innovation does happen without IP. It is possible to have both access and innovation, we just need to reconceptualize how pharmaceutical research and development is structured.
This paper makes a concerted effort to do that. Here, we make the case for urgently reforming the current system of medical innovation, highlighting how such a system is unsustainable and undesirable in the long term. We propose a new system, one which rewards medical innovation and access separately. This system, based on a remuneration right similar to a patent, rewards medical innovators whilst allowing free and open access to innovations so they can be produced at cost by manufacturers and other innovators can build upon their ideas.
We demonstrate that such a system is politically and technically feasible, and, most importantly, immensely desirable: it will expand access to medicines for millions in both developed and developing countries and help align innovation efforts more closely with actual health impact.
We show that many of the necessary precursors of such a system are already in place, and that the costs of implementation would be significantly outweighed by the benefits in improved access and innovation.
Ultimately, and in spite of its historical successes, the current system of medical innovation does not work. It necessarily limits access to essential medicines and, in many cases, fails to incentivize the kinds of development we want as a society. The unsustainable trajectory of this system means it is imperative to search for new mechanisms of funding medical innovation, moving beyond the ingrained trade-off between access and innovation a monopoly rights patent system engenders.
Remuneration rights do just this, and by dividing payment for drug access and drug innovation, we can refocus our medical priorities and greatly expand access at the same time. This is a huge opportunity; we can have both access and innovation at the same time and all we need to do is innovate in how how we pay for medicines. Under such a system, everybody wins.
Pharmaceutical research and development (R&D) has delivered some of the greatest achievements of the modern age: the polio vaccine, antiretroviral drugs to combat HIV, and insulin. But the system for funding R&D is broken, and heading for catastrophe. Millions of people do not have access to medicines because of the high prices charged to recoup the costs of research, and many urgent health problems go without treatments because of the lack of a profitable market. Simultaneously, profitable but insignificant areas are over-invested in. Moreover, there are structural blockers in the form of legal disputes and licensing restrictions which hinder innovation and delay us receiving the most cutting edge of medical innovation. If current trends in prices and innovation continue, these problems will worsen over coming years and become untenable. It is therefore essential that we find solutions to them now.
Rising prices combined with an aging population, demographic growth and falling therapeutic benefit make for an unsustainable system. The past decades have seen an increase in the price of medicines, and a decrease in the therapeutic benefits associated with new innovations.2 Projections suggest these trends will continue. It is therefore imperative to take action before the price of healthcare becomes completely unsustainable, and the number of new and universal medicines dwindles. Governments will not be able to afford to buy medicines for their citizens at this rate.3 Such an outcome would not only spell disaster for state budgets and ordinary citizens, but also for pharmaceutical companies’ profits. Bankrupted healthcare providers will no longer be able to purchase from pharmaceutical companies if prices rise indefinitely. Everyone has a stake in changing the system to preserve both rewards for innovation and access to medicines.
The prices of medicines are eye-watering. According to the Express Scripts Prescription Price Index, branded drug prices doubled between 2008 and 2016.4 As the Kaiser Family Foundation has demonstrated, using data from the National Health Expenditure Account, United States (US) spending on prescription drugs has been on a steep incline for decades, even when adjusted for inflation (Figure 1). In 1980, \$30 billion was spent, adjusting for inflation; in 2016 this figure soared to \$329 billion - an increase of over 1000%. Recent years have seen particularly steep increases, with expenditure in 2015 at 120% of that just two years previously in 2013.5 In 2017 alone, pharmaceutical company Pfizer raised the price of 91 of its drugs by an average of 20%.6 Such price hikes are now threatening access in even the richest of countries.
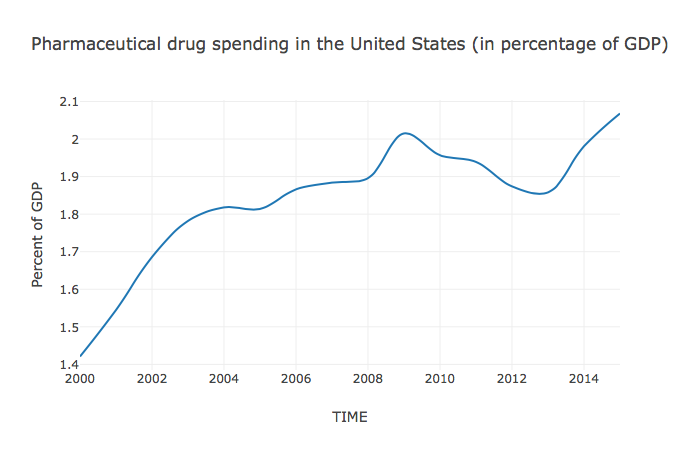
Source: “Health Spending Explorer.” Peterson-Kaiser Health System Tracker (blog). Accessed October 25, 2017. https://www.healthsystemtracker.org/interactive/.
As well as getting more expensive, drugs are also getting less effective. Figure 2 shows the results of the annual review of new products and new indications for drugs already on the market undertaken by Prescrire International. Just 16% of new drugs in 2016 had even a possible advantage over their predecessors, compared with 42% in 1992. The absolute number of drugs with possible therapeutic advantage has also fallen, from 60 new drugs in 1992 to just 15 in 2016. Meanwhile, in the same time period the percentage of drugs offering no additional therapeutic benefit rose from 53% to 78%. If such trends continue, we will not only face unsustainably high drug prices in the future but also a grave shortage of effective drugs.
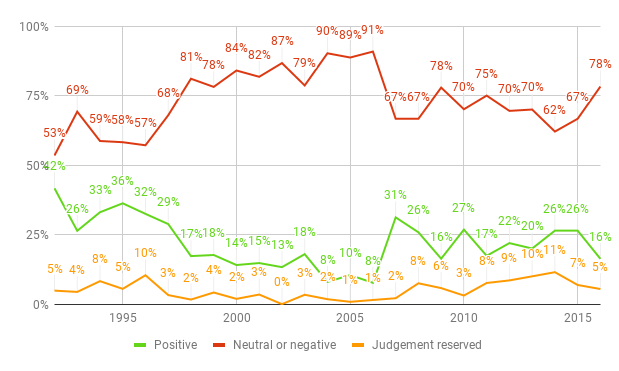
Sources: “A Look Back at Pharmaceuticals in 2006: Aggressive Advertising Cannot Hide the Absence of Therapeutic Advances”, p. 84; “New Products and New Indications in 2016: A System That Favours Imitation over the Pursuit of Real Progress”, p. 138.
Millions of people today do not have access to the medicines they need. The World Health Organisation (WHO) estimates that around a third of the world’s population does not have access to medicines.7 There are many reasons for a lack of access to medicines: availability, affordability, appropriate use, and drug quality.8 Affordability, a combination of price, cost and availability of funds, is a primary concern.9 This particularly affects the poor and those living in poorer countries. In wealthier countries, governments have also become unable to meet the rising costs of new medicines. Consequently, millions of people go without treatment or experience delays in doing so. This has obvious costs to the individual, but also serves to place additional strain on public healthcare systems and reduces economic productivity.
While it is difficult to find estimates on the lack of drug access globally, there are numerous cases that illustrate the low levels of access to medicines today. The WHO estimate that in 2008, 8.8m children died from vaccine-preventable illnesses.10 In 2017, the WHO claimed that 1.5 million deaths could be prevented annually if vaccination coverage improved.11 Figure 3 shows access figures for three high-burden diseases: Human Immunodeficiency Virus (HIV), hepatitis C and tuberculosis (TB). The numbers of people who currently go untreated for these diseases is staggering: 16 million with HIV,12 66 million with Hepatitis C, 13 and 4.1 million with TB.14 It is unclear exactly how many of these people would be able to receive treatment at close to marginal cost, but even with very conservative assumptions, it is clear that many millions are unable to access medicines because of unaffordably high drug prices. This is especially important given the threat to global health security posed by conditions like HIV and multiple drug-resistant TB. Everyone benefits from increased access to medical treatment, not just the poor or the sick.
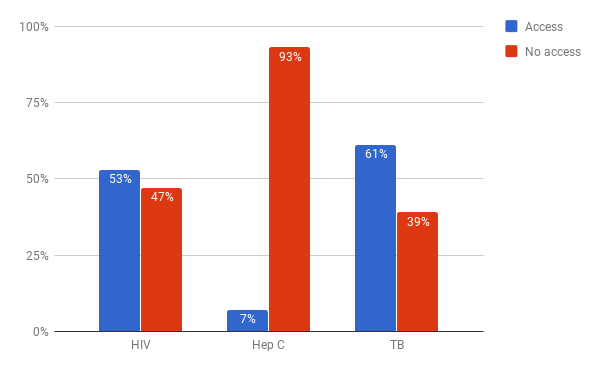
Sources: “GHO | By Category | Antiretroviral Therapy Coverage - Data and Estimates by Country.” WHO. Accessed October 25, 2017. http://apps.who.int/gho/data/node.main.626; “GHO | By Category | Treatment Coverage - Data by WHO Region.” WHO. Accessed October 31, 2017. http://apps.who.int/gho/data/view.main.57056ALL?lang=en];“Global Hepatitis Report, 2017.” World Health Organisation, 2017. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1; “WHO | Hepatitis C.” WHO. Accessed October 25, 2017. http://www.who.int/mediacentre/factsheets/fs164/en/.
These systemic and persistent failures inaccess are directly caused by the patent system. Patented medicines are produced exclusively and without market competition, consequently being sold at prices far above the costs of manufacture. Figure 7 in Section 6.2 gives examples of the huge gap that is often present between the cost of manufacture and the sale price of a medicine. This gap exists to compensate for high R&D costs. Under the patent system, such high prices are not just likely: they are necessary. The way that the patent system funds innovation, which we need if we are to continue to have new medicines developed, is through high prices today, restricting access for those who are sick in the here and now.
In spite of the incentives for innovation generated by patents, there are a number of serious problems with the way in which innovation operates today. A critical role of the pharmaceutical industry is to create new medicines which benefit society, but equally vital is to make these products available to those who need them. The current patent system is not achieving this requirement. Because financial incentives for innovation are tied to the market, unprofitable but important areas of health are neglected; simultaneously, profitable but relatively insignificant areas in terms of health impact are heavily invested in. Finally, there are structural blockers to research , such as patent disputes and licensing which unnecessarily delay and complicate the development of new medicines.
Ultimately, the reason that pharmaceutical innovation matters is for people to be healthy. Pharmaceutical companies are motivated by this philosophy, but they also have a responsibility to their shareholders. Yet profitability is a poor proxy for health, especially given wealth inequalities. Many of the most widespread and serious diseases primarily affect the poor (e.g. malaria). As the people who suffer from these diseases cannot afford to pay large amounts for medicines, the market does not provide a sufficient incentive for pharmaceutical companies to conduct the necessary R&D. A similar effect can be observed with chronic and acute diseases: as those with chronic illnesses continue to need medication, it is more profitable to invest in research for chronic needs than acute but serious illnesses. This means that important areas of health are systematically neglected in the R&D pipeline.
Neglected diseases include malaria, TB, diarrheal diseases, and tropical diseases; they are defined as diseases that affect people in low-income countries and are a leading cause of mortality, chronic disability and poverty. Over a billion people live with one or more neglected tropical disease.15 In 2010, “Only about 1% of all health R&D investments were allocated to neglected diseases.”16 And “in 2013, public and private investment for R&D in 34 neglected diseases was \$3.2 billion, of which pharmaceutical corporations only contributed \$401 million. The latter amount represents only 0.8% of total industrial R&D spending of \$51.2 billion in 2014”.17 The global landscape of health R&D shows a substantial gap: diseases of relevance to high-income countries were investigated in clinical trials seven-to-eight-times more often than were diseases whose burden lies mainly in low-income and middle-income countries.18 Monopoly patents do not offer a sufficient incentive to innovate in these areas, because the people affected by these diseases are mostly poor. In a system where profit is tied to sales, drugs to treat these patients will not be developed. This has a huge human cost for those who suffer from such diseases, and also poses risks to the world at large. Unchecked tropical diseases increase the risk of global pandemics like ebola, while entrenching poverty and so increasing global instability. It is better for human society if leading causes of mortality and morbidity are treated.
On the other hand, profitability does incentivize areas of research which have low health impact, or in some instances no health impact at all. The profit incentive is insensitive to how large a therapeutic benefit a treatment will have. Provided that a sufficiently large or sufficiently wealthy market exists, there are incentives for R&D. This leads to areas of low therapeutic significance being over-researched - a poor allocation of resources from the point of view of social welfare.
It has been estimated that much of the neglected tropical disease burden, which impacts 1.4 billion people in South Asia and Sub-Saharan Africa, could be alleviated for just \$300 million to \$400 million a year.19 However, the current mechanisms for rewarding medical innovation simply do not provide avenues for the return on investment for this kind of research.20 It simply does not happen, or does so in a much diminished capacity, often requiring state or philanthropic support. In fact, much more money is spent each year on the cosmetic anti-ageing market: the global anti-aging market was valued at \$140.3 billion in 2015,21 in spite of the limited health impact it generates.
Provided a drug can be sold legally and to a sufficient extent, it is profitable to make, even if it does not represent any real improvement on existing drugs.22 In 2005, Love and Hubbard claimed that “probably one-half to two-thirds of the R&D investments were directed towards projects of almost no medical significance”.23 And “an analysis of 1345 new medicine approvals in Europe revealed that no real breakthroughs occurred between 2000 and 2014; only 9% of new medicines offered an advance, and 20% were possibly helpful”.24
Alongside these problems of allocation, there are also structural blockers to research of all kinds under the patent system. Patents are a monopoly rights and are explicitly designed to limit anyone other than the innovator from using the information or accessing the knowledge behind an innovation. Those who do not follow these rules are harshly penalized. As a result, costly management and infringement settlements are pervasive.25
Legal disputes can stall innovation for many years, preventing new medicines from reaching the public. Historical examples include the telephone, the radio and the automobile.26 Moreover, failure to obtain a license from a patent holder means that potential innovators cannot proceed. A good example of this is the monopoly over breast cancer genes formerly held by Myriad Genetics. Until the patent was revoked, further research and more widely available and affordable testing were stalled.27 In contrast, the opening up of intellectual property has proven to be effective boosters for innovation. In the aftermath of the First World War, compulsory licensing of German chemical patents in the US significantly improved innovation there.28 This indicates that patent enforcement can slow down R&D.
There are good reasons we use patents: they provide effective incentives for innovation, ensuring that innovators are rewarded. Simply voiding patents, as some people have argued, is unlikely to be a viable solution either practically or politically. If patents were simply to disappear private investors would be left with little incentive to undertake expensive R&D at all, and future generations would see a deficit of new medicines.
Unfortunately, the patent system also creates a fundamental tension between access and innovation. More access means less innovation and vice-versa. With patents, high prices are needed to fund innovation but high prices mean fewer people can be treated (crucially, the lost purchases of those who cannot afford treatment benefit no-one as the patients remain sick and the pharmaceutical company gains no revenue). Conversely, lowering prices for medicines to increase the number of patients with access would mean less money for pharmaceutical companies to invest in innovation.
As a society we obviously want both access and innovation. At an extreme, one is practically worthless without the other: imagine a world in which everyone has access to what medicines there are, but there are virtually none available; conversely, imagine a world in which all diseases have a cure but no one can afford them. The patent system trades these two things off against one another. Without systemic change, there is no way around this fundamental dilemma: either one increases access and decreases innovation, or one increases innovation and decreases access.
Given the ubiquity of the patent system, and a broader culture of protecting intellectual property29 this trade-off can seem natural or inherent. However, the trade off is merely an artefact created by the nature of patents as a funding mechanism. Medicine is made up of two parts: information and manufacture. The information part of medicine is intangible, produced through R&D, and extremely expensive. The manufactured part of medicine is physical, produced in factories and relatively cheap to make. Patents create a single payment for these two elements of medicine: both R&D and the manufactured pill are paid for by the consumer, be they a government, an insurer or an individual patient. Because patents combine the payments for these two elements, the cost per usage of a given treatment must be high to cover the R&D. This means that the number of treatments that can be afforded by a given healthcare consumer is limited, and many are denied access as shown in Section 2.2. And this denial of access is necessary under the patent system: otherwise R&D will not be incentivized in future.
But it is possible to pay for R&D and manufacture separately, thus resolving the conflict. By splitting the single payment of the patent mechanism into two, the price of each individual unit of a medicine can be drastically lowered, while maintaining high incentives for innovation.
We need a new approach at this critical juncture. A point where millions are unable to afford access to essential medicines and where even in the richest countries soaring prices are leading to rationing and a crisis in funding. We need a two-part payment system that pays separately for innovation and manufacturing medicines delivering both high levels of investment in innovation and high levels of access to affordable medicines. Remuneration rights do just this
We propose an alternative kind of property rights,30 remuneration rights, as the best alternative to the patent system for medical innovation. Under remuneration rights, innovators would obtain a non-monopolistic “remuneration right” rather than the monopoly patent they receive today. Remuneration rights entitle their owner to payment from a central fund according to the value generated by the innovation: how much a given drug improved health. In return, the innovation would be open for use and manufacture by everyone, improving access and further facilitating innovation.
Figure 4 depicts the core aspects of the remuneration rights model. Pharmaceutical companies and other innovators undertake R&D and create new medicines. Innovators register for a remuneration right instead of a patent. In exchange for a remuneration right, all R&D has to be freely available to any certified manufacturers to produce. This enables competition among manufacturers to make high quality, cheap medicines. Healthcare buyers purchase these medicines at prices close to the cost of manufacture. Lower prices per pill dramatically increases patient access to medicines. Meanwhile, citizens continue to pay taxes to their governments. Governments pass a small proportion of their health care budget to health care buyers to purchase medicines. A much larger proportion is paid into an independent remuneration rights fund. This fund disburses its funds on an annual basis to the owners of registered innovations. Each innovation is rewarded in proportion to the health impact it produced.
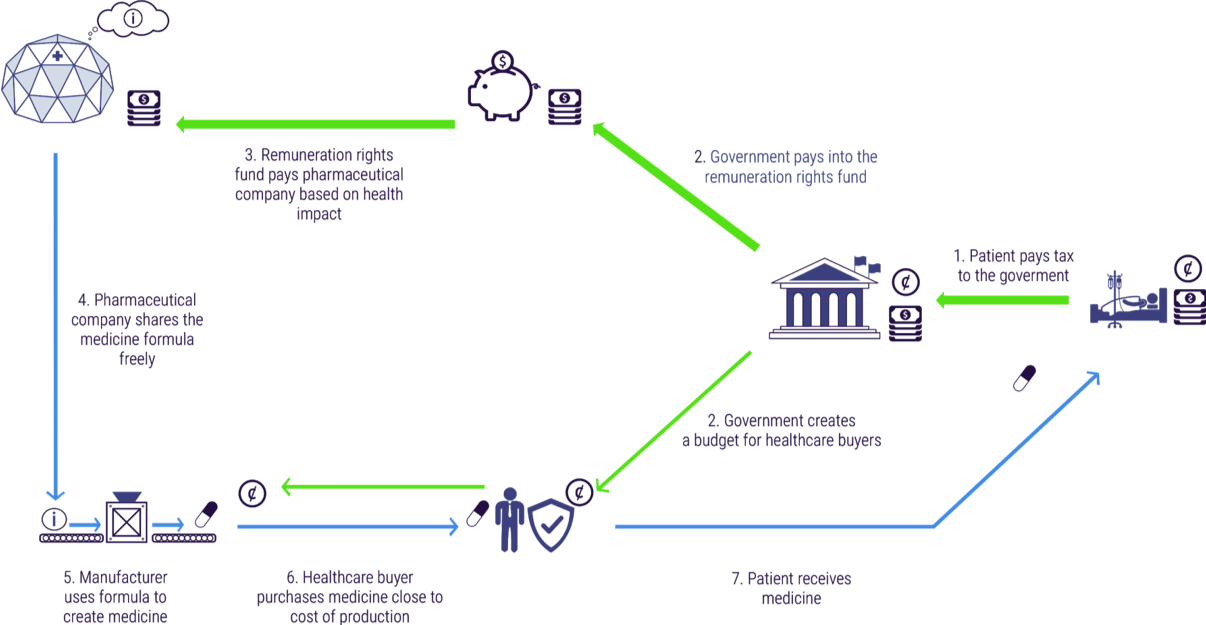
Crucially, under the remuneration rights system, medicines and R&D are paid for separately. Currently when we pay for a medicine we are paying both for the expensive R&D (perhaps billions in total) and for the cheap cost of manufacturing (perhaps just a few dollars per pill). As an example, when we pay \$50,000 for a medicine perhaps 99% (\$49,500) is going to pay for the R&D and 1% (\$500) is paying for the manufacturing.
With remuneration rights, the billions of R&D cost are paid for via the remuneration rights fund, and the hundreds of dollars to manufacture the medicines are paid through current channels of drug purchasing. Because the price of medicines is close to the cost of manufacture, people can get the treatment they need. Meanwhile, innovation is still paid for and incentivized through the remuneration rights fund.
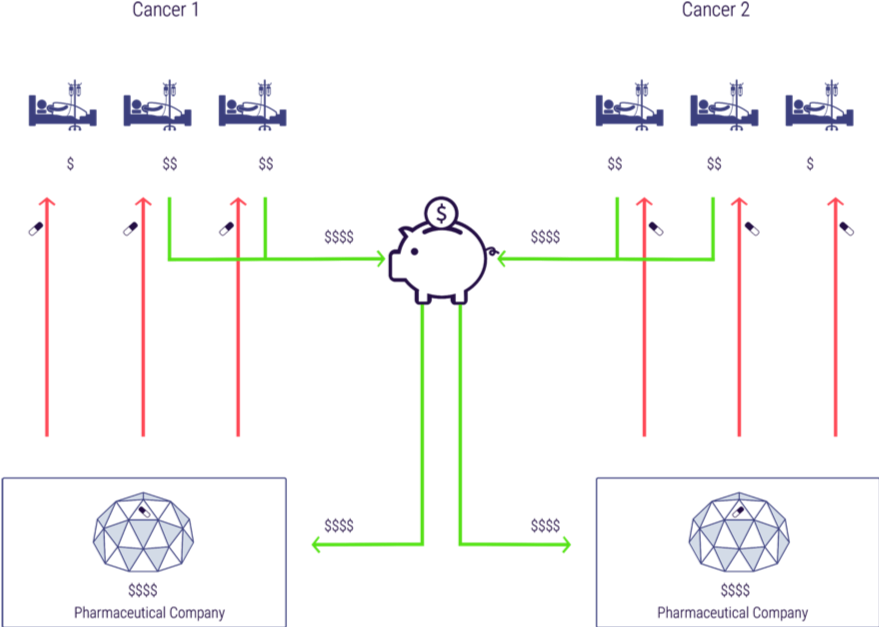
Figure 5 shows how medicines and monies would flow under the remuneration rights system, using the example of a simplified world with two cancer drugs. Patients in this example (all insurees/taxpayers normally) would contribute to the remuneration rights fund, which would disburse money to innovators to pay for R&D (the two richer patients in the diagram with \$\$). Competition in manufacturing would mean the medicines would then be available to all patients and monies from the fund would be distributed to the relevant innovators proportionately to use and impact (in this case we assume the benefit of each treatment is the same and so the monies are distributed to the manufacturers 50:50). In this case the ultimate distribution of monies to innovators is exactly the same under remuneration rights as under patents but in contrast to the patents case all three patients receive treatment. (Note, for simplicity we have omitted manufacturers from this diagram as we are seeking to illustrate the distribution of monies to innovators).
We already have substantial sources of funding for medicines in the form of healthcare budgets, divided amongst direct grant giving and indirect funding through drug purchases. This funding comes from taxpayers, employers and insurers. Under a remuneration rights system, these groups would continue to contribute healthcare payments as today. In government-based health systems, this money would come primarily directly from taxpayers and employers. In insurance-based health systems, much of this money would be paid first in insurance contributions, and then transferred on by insurance companies.
Governments would pool existing contributions into an independent remuneration rights fund. There are a number of ways the level of funding might be determined. One way would be to set a percentage of GDP for each participating government to contribute. Another would be to set the size of the fund at the current level of private spending on pharmaceutical R&D, and grow the fund at the current level of growth in private research spending. The fund would have a fixed disbursable pool available for innovation funding each year. Governments would be legally bound to fulfil their respective contributions. Once governments had deposited their funds, they would no longer have control over the allocation of the monies, ensuring the independence of the fund.
Innovators would apply for a remuneration right from a remuneration right office, similar to patent offices today. This right would not grant market exclusivity, and would enjoin open access to the information behind the innovation. Instead this right would entitle innovators to be paid from remuneration rights fund based on the impact of their innovation on health.
Specifically, each year, the disbursable funds in the remuneration rights fund would be divided up among the holders of remuneration rights, in proportion to the health impact they created. Where innovators build upon the ideas of others, a portion of their remuneration right would be set aside like a royalty, to be delivered up to the originators of the relevant innovations. Health impact would be estimated using a predetermined and transparent metric. There are several examples of these metrics today, like the Quality-Adjusted Life Year (QALY) or Disability-Adjusted Life Year (DALY), which are used by institutions like the National Institute for Health and Care Excellence (NICE) and the WHO.
In this way, remuneration rights have a strong market-like aspect: payment is determined by individual usage and its benefits. In some respects they are similar to patents, but without the disadvantage of creating monopolies. And just as patents have a limited term, remuneration rights would eventually expire. This would ensure that money continued to flow towards newly invented treatments, and would reduce the need to keep track of the impact of older treatments, which might be infrequently used or simply superseded.
Access would increase dramatically at no cost to innovation and with little or no increase in spending. Citizens would pay their tax (or insurance contributions in insurance based systems) just like today. There might be small additional cost of additional manufacturing as lower prices mean we choose to expand access. The overall result is vastly increased access to medicines for patients.
The way in which patients receive medication would remain the same - but the price to themselves, their insurers or their health system would fall per treatment. Given that millions of people currently do not have access to essential medicines, this could mean a huge increase in health outcomes for a small additional investment.31
=
There are many important groups involved in the funding, research, development, manufacture, distribution and use of medicines. Remuneration rights would change the experience of some groups operate, while leaving that of others little changed. This section sets out in detail the implications of the remuneration rights system for some of the most important stakeholders in the medical ecosystem.
Patients receive medicines in the same way as today, but more treatments are available to them at much cheaper prices.
Patients would continue to use prescription medicines paid for by the parties currently responsible, be they government, insurance companies or patients themselves. What would change for patients under remuneration rights is that more treatments would be available. Currently, expensive treatments are rationed or not provided at all in many countries. Separating the cost of the medicines themselves from the cost of the R&D would mean that each additional treatment, rather than costing hundreds of thousands of dollars, might cost hundreds or even less.
Healthcare professionals continue to prescribe as today, but with access to greater treatment volumes.
Doctors, pharmacists and other healthcare professionals would continue to prescribe medicines as they do today. However, they would have access to many drugs which are currently too expensive to prescribe at all, or in all but the most serious cases.
Healthcare buyers can purchase medicines from a greater range of manufacturers at greatly reduced prices.
Healthcare buyers would be able to choose between a wide range of manufacturers when purchasing medicines. This would give them greater power to negotiate prices, which in any case would be driven far lower than today thanks to competition in manufacture.
Civil servants implement the fund according to predetermined, predictable and objective guidelines.
Civil servants would implement the transition to the remuneration rights model, and would then operate the fund itself. This would be a significant task for countries participating in the remuneration rights fund. The nature of remunerating innovations based on health impact is more technical than discretionary, which enables transparency and predetermination of selection criteria.
Politicians can deliver much greater health care and remain independent of decisions about health impact.
Politicians would be able to deliver greater health care for only a comparatively trivial increase in funding (to cover the low cost of manufacture for additional physical medicines). Meanwhile, they would remain independent of decisions about health impact, allowing these decisions to be made without undue political influence and sparing politicians hard decisions between treatment types.
Employers pay health care contributions as today, and their employees can access much greater levels of treatment for similar cost.
Employers would continue to pay insurance contributions for their employees, either at the same or a very slightly increased rate (to cover the low cost of manufacture for additional physical medicines). Their employees would receive far greater levels of access to medicines in exchange for this similar input. This can be expected to increase productivity , minimize sick days, and boost employee wellbeing.
Insurance companies pay into the fund, and their insurees can access much greater levels of treatment for similar cost.
Insurance companies would pay contributions into the fund. Their insurees would receive far greater levels of access to medicines. Meanwhile, insurers would benefit from the vastly reduced price of medicines. Overall, insurers would pay slightly more (to cover the cost of manufacture for additional pills) for greatly increased health coverage.
Taxpayers would continue to contribute to national health budgets. The slightly increased expenses of the system (in paying for the manufacture for many more pills) would mean slightly more money spent on healthcare (though this could potentially be offset by efficiency gains). In exchange, taxpayers would benefit from drastically increased access to medicines.
Shareholders continue to receive profits from pharmaceutical companies.
Pharmaceutical companies would remain profitable businesses, but their profits would be tied to health impact rather than to pure sales. Shareholders would still receive these profits. Furthermore, the structure of the remuneration rights fund would deliver greater certainty to investors about the long-term revenues available to the industry.
Marketers continue to market drugs. Marketing undertaken by manufacturers feeds into profits for the owners of the remuneration right.
There would still be a need to market drugs. Although profits would no longer be tied directly to sales, as health impact is related to the number of patients treated, there would be an incentive to market effectively. Competing manufacturers would use marketing to increase their market share. Ultimately, innovators would benefit from marketing undertaken by manufacturers: it would increase the use and therefore the health impact of their innovation, and so lead to a greater payment from the remuneration rights fund.
Distributors have an expanded remit, as rewarding innovation based on health impact provides a financial incentive to distribute to hard-to-reach patients.
Distributors of pharmaceuticals would experience vastly increased incentives to reach more marginal patients. Currently there is only an incentive to distribute to wealthy markets, as remuneration is based on sale price alone. Under the remuneration rights system, as remuneration is tied instead to health impact, there is a stronger incentive to distribute to poor markets too.32 This would encourage distributors to expand into emerging markets, as well as increasing global access to medicines.
Qualified manufacturers can produce any medicine in competition with other manufacturers.
Manufacturers would have license and royalty free access to any innovation registered with the remuneration rights office. This would expand the opportunities for manufacturers whilst ensuring high quality production at competitive prices. Manufacturers would still be subject to extensive quality control, so drug quality would be maintained.
Innovators file for remuneration rights instead of patents and are paid annually from the fund.
As producers of information, innovators would file for a remuneration right. They would then be entitled to a share of the remuneration rights funding pool for a fixed period of time. Payments would be made annually. The payments to the rights holder would be proportional to the usage and health benefits of the treatment.
As consumers of information, innovators would have unrestricted access to research and information on all innovations registered with the remuneration rights office. This would reduce duplication and accelerate cumulative research.
Investors get returns from R&D that delivers health impact.
Investors continue to receive returns on investments in pharmaceutical R&D. The only difference is that the most successful investments would be in the innovations which produce the most health benefit, rather than in the innovations which sell best.
Basic researchers continue to be funded through research grants.
Basic researchers would still be funded through research grants, as today. Grants are a push funding mechanism, and fund research upfront, rather than after the fact.33 This is the most suitable way to fund early stage research, as it permits exploratory work into new research areas, without predetermining specific outcomes.34
In order to represent a viable alternative to the patent system, remuneration rights must be technically and politically feasible to implement. Technically, many of the aspects required under a remuneration rights system already exist; we already have robust means of measuring health impact, the patent system requires ways of defining innovation ownership as well as what happens when patented innovations are built upon by others. Each of these mechanisms could be reused for remuneration rights.
Furthermore, much of the political infrastructure required for a remuneration rights system is already in place, including coherent international (and often national) legislation and means of arbitration that could be co-opted, similar governing bodies for related funds, and the means of securing sustainable funding. This means there are few significant barriers to a transition to a remuneration rights system.
For remuneration rights to be a viable funding mechanism, the technical aspects of the model must be practicable. Three issues stand out:
Delimitability. Remuneration rights require the determination of which innovation belongs to which innovator.
Reuse. As research is cumulative, it is important that the remuneration rights model can reward innovators who build on the work of others in a proportionate manner, while not disadvantaging the originator.
Health impact measurement. Health impact must be measurable in a reasonably accurate way for remuneration rights to be allocated appropriately.
Fortunately, there are precedents for all three of these technical features.
It is crucial to both the patent and the remuneration rights system that innovations can be separated from one another. In order to give a right, whether a patent monopoly right or a remuneration right, to an individual, we have to be able to attribute innovation correctly. This process is vital in the patent system, could be substantially reappropriated under a remuneration rights system.
Because it aims to confer special privilege over a particular innovation, the question of delimitation is central to the patent system and therefore well defined within that framework. When submitting a patent application, the goal for the innovator is to demonstrate that the product (or treatment) represents a significant innovation originating from the applicant. The application process is itself a delimitation exercise which defines the innovation.
Remuneration rights cover the same sorts of innovation patents do, and would be delimitable in the same way. To qualify for a remuneration right, innovators would have to prove that they were the originators of a particular medicine or treatment in the same way they currently do when applying for patents. The infrastructure of the current system can to a large extent be maintained: the patent office would simply become the remuneration rights office. Aside from the end product, very little would change.
Remuneration rights would be granted on the condition of completely open access to all information relating to the innovation. This would enable innovators to build on previous ideas more easily. It is therefore important that remuneration can be shared fairly between originators and innovators who build upon these.
This already happens in the patent system, albeit to a more limited degree. Because research is cumulative, reuse is frequent and important in medicine, although it is inhibited by the blockers discussed in Section 2.3.3. Under the patent system, follow-on innovators are required to pay royalties to the original innovator.
Within the remuneration rights model, anyone would be free to build on the work of others. In a similar fashion to royalties in the patent system, follow-on innovators would be liable to pay a proportion of their own remuneration rights payments to those whose work they built upon. These proportions might be standardized for simple cases or, for more complex cases, the two parties could negotiate, with ultimate recourse to the courts if no mutually acceptable solution were found. In other words, if an innovation built upon a previous innovation holding a remuneration right, then a proportion of the right granted to the secondary innovation would be set aside for the initial innovator(s).
The major difference with the patent system would be that the original innovator would not have an absolute right to prohibit reuse as they do today. Rather, they would have the right only to \“equitable remuneration\”. This change would favor the follow-on innovator, while ensuring that the originator was fairly compensated. The remuneration rights model would therefore make it easier for innovators to build on and incorporate previous work, preventing the delays seen today.35 The system would operate along the same lines as the patent system today, but without the right to prohibit reuse.
Remuneration rights depend on the measurability of health impact. While any measurements would be inexact, there are several good measurement systems which could be deployed, some of which are already in use today.36 Some jurisdictions already use these to determine their medicine purchases: for example, NICE in the United Kingdom (UK) uses QALYs to decide whether or not a medicine is cost-effective.37 The remuneration rights fund would extend the methods and data gathering procedures that already exist, creating a rich pool of information for improving healthcare in general while simultaneously rewarding innovation more fairly.
There are several sources of data that would be used to assess the health impact of a particular innovation. Initially, clinical trial data would be used as a baseline for the efficacy and therapeutic benefit of a drug. Today clinical trials showing safety and efficacy are already required for all new pharmaceuticals so this data is already being collected. As time passed, observational data would become available on the actual benefits caused by the medicine. Additionally, we also already track prescribing data so that the number of treatments given out can be calculated.38
No health impact assessment will be perfect. Data will be incomplete, and some things will be difficult or controversial to quantify. But health impact assessment need only be accurate enough that the best strategy for companies seeking remuneration rights is to actually produce health benefits.39 Moreover, because companies are competing for a fixed amount in the Remuneration Rights Fund for Medicines they have an incentive to hold each other account and check fraud and abuse (if company X fraudulently overstates the use and health impact of their treatment this reduces the monies for competing company Y).
As well as functioning technically, remuneration rights must be able to operate politically. This includes the sustainable financing of the fund, the fund’s governance structure, the relationship between the fund and governments, and the legal status of the fund.
For the fund to function reliably on a global scale, two essential conditions must be met: international agreements binding all countries to contribute to the fund, and a systematic way to set equitable contribution levels must be agreed upon.
Before pooling contributions into an independent remuneration rights fund, it is essential to prevent potential free riders from benefiting from the efforts produced under the remuneration right model, both within and between countries, without contributing to the fund themselves. A remuneration rights fund without such safeguards against freeriding would be unsustainable. This could be done by establishing international agreements under which countries bind themselves to minimum levels of medical research funding, as happens with military spending. Governments would be legally bound to fulfil their respective contributions, which would ensure the fund would have a fixed disbursable pool each year. Once contributions were deposited, governments would no longer have control over the allocation of the monies.
The size the fund of could be determined in two different ways. First, it could be benchmarked on the the current level of private spending on pharmaceutical R&D, and grow at the current level of growth in private research spending. In the US, for instance, current levels of public and private research funding together amount to \$100bn a year (depending on exactly how medical research is demarcated). Alternatively, each country could agree to allocate a given percentage of their GDP, perhaps 0.5% to 1% of GDP, raised primarily out of general tax revenue. As an illustration, 1% of GDP for the United States would amount to about \$186bn a year.40 In time, the fund would need to be adjusted to follow inflation and demand for healthcare. The level could also be adjusted to reflect the level of development between countries, with richer countries committing themselves to higher proportions.
The fund would be governed by an independent body. Stakeholder participation would be important to ensure that participating states are willing to join the remuneration rights fund.41 Transparency would also be vital, to create trust in the operating of the fund. Ultimately, the most important feature of the governing body would be its impartiality. One way of achieving this would be to make governance independent of electoral politics and political faction. Another might be to separating the performance of health impact assessment from the establishment of guidelines for the same.42 This would minimize the discretionary function of health impact assessment and reduce the risk of external influence being exerted.
Specific governance designs have already been proposed by previous proposals operating along similar lines to a remuneration rights fund:43
The Medical Innovation Prize Fund Act (MIPF), submitted most recently to Congress in 2017 by Bernie Sanders, proposes a national remuneration rights-style fund for medical research in the US.44 The system would be administered by a board of trustees and six expert advisory boards. There would also be a system of competitive intermediaries, who would compete for funding and allocate some of the rewards.45
The Health Impact Fund (HIF), proposed by Aidan Hollis and Thomas Pogge in 2008, would be a voluntary, global remuneration rights fund:
“The HIF will be governed by a Board of Directors chosen by funding partners, exercising primary responsibility over the Fund. The Board will oversee three branches representing the core functions of the Fund: the Technical Branch, the Assessment Branch, and the Audit Branch. These will, respectively, set the standards for evaluation of health impact (Technical), determine individual products’ actual impact (Assessment), and ensure correspondence between standards and evaluations (Audit).“46
Such designs would need to be considered in a feasibility study. Our proposal builds upon these previous models.48
The remuneration rights fund would operate in a global context, liaising with many governments. There are questions about both cooperation between participants of the fund and between the fund and non-members. The most crucial point of tension in implementing and maintaining a remuneration rights system will be how to negotiate the potential issue of free riders, whereby non-members enjoy the benefits of open-access without contributing towards R&D costs.
There are questions remaining as to whether the fund could operate successfully on a national level or whether wider international cooperation would be required. There have been proposals for national remuneration rights-style funds like MIPF,49 and a pilot study might well be national in scale. Other advocates think that a minimum viable level of participation pertains. For instance, the proponents of HIF regard the minimum threshold to be a fund comprising states representing a third of global income.50 Whether the fund is national or international in scope, participants would need to contribute sufficiently to encourage innovation. To obtain the full benefits of the fund, member states would also need to have a lively manufacturing sector; generic competition tends not to lead to marginal cost unless there are 10 or more competitors.51
At least initially, there would likely be states operating outside of the remuneration fund. This raises the question of non-members exploiting the open-access nature of the fund without contributing towards it, and of members requisitioning patented innovations elsewhere, to manufacture internally, without compensating patent holders. Core to governing this relationship would have to be a mutual recognition of patents and remuneration rights. Members of the remuneration rights fund would continue to respect the IP rights of non-member states and alternative means of payment may need to be developed for non-members wishing to use innovations in the fund.
International treaties, differential pricing agreements or some other means of translating between the two systems will need to be established to allow a successful coexistence of both systems.
There is extensive national and international legislation covering the patent system. The remuneration rights model is compatible with these legal frameworks. The most important of these is the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS), which is compatible with the remuneration rights system.
The patent system, while still defined and enforced in national jurisdictions, has been internationalized through the TRIPS agreement. TRIPS governs nearly all aspects of intellectual property in international trade. Prior to TRIPS, different countries had different patent laws, which often reflected their level of development and the social goals that patent laws were thought necessary to achieve. Today, TRIPS requires all World Trade Organisation member states to maintain strict patent protection laws for patented pharmaceuticals, with a guarantee of at least 20 years of market exclusivity.
While the patent system enforced by TRIPS is global and binding, it also has built-in flexibilities. Legal provisions are in place to allow exceptions to exclusive rights in order to widen access and bypass the patent monopoly under specific circumstances. Referred to as “compulsory licensing”, these flexibilities allow the use of patented innovations without the consent of the owner in cases of (public) non-commercial use, national emergencies, other circumstances of extreme urgency, as well as in anticompetitive conduct. Article 30 of TRIPS clarifies that exceptions to exclusive rights must be limited, must “not unreasonably conflict with a normal exploitation of the patent,\” and must “not unreasonably prejudice the legitimate interests of the patent owner, taking account of the legitimate interests of third parties”. Provided that a remuneration rights fund where set at a sufficiently high level, it would therefore qualify as a legitimate exception, as it would take the interests of innovators into account through sufficient remuneration.52 These flexibilities are already in place, legal and would allow remuneration rights to function without requiring additional legal frameworks to be created.
Other examples of TRIPS compliant (and legal), but non-patent-based, funding mechanisms exist. Prize funds are a good example. For instance, the Longitude Antibiotics Diagnostics Prize offers \$10m for a diagnostic test which helps to conserve antibiotic use.53 Patent pools are also used today to increase access to medicines. The Medicine Patent Pool (funded by UNITAID) aims to reduces the cost of drugs treating HIV, viral hepatitis C and TB by pooling the licenses required for future development, making it it easier for innovators to create new drugs. They negotiate directly with pharmaceutical companies to encourage them to voluntarily join an HIV patent pool.54 Thus, generic manufacturers can obtain licenses more easily, increasing competition and reducing prices.55
Overall, remuneration rights would be legally compatible with existing international law governing intellectual property. There are also mechanisms on a national level that would allow for disputes to be resolved. Remuneration rights could use existing intellectual property legislation for dispute resolution. In case of a disagreement on remuneration allocation, parties could apply for arbitration to the courts, similar to the patent system today.
Sections 4.1 and 4.2 have shown that a remuneration rights system would be feasible. There is also a question of whether it would be feasible to transition from the present patent system to a remuneration rights model. Firstly, many aspects of the current funding system would remain unchanged under remuneration rights, making the transition less traumatic. Secondly, there is the option of having remuneration rights coexist with patents, permanently or for a transition period. Finally, transitions of this scale have happened in the past without deleterious effects.
Many aspects of the current funding landscape for pharmaceuticals would remain unchanged under a remuneration rights model. Figure 6 sets out the main institutions of the remuneration rights model, with their equivalents under the current system. First, academic-style funding for research would continue largely unchanged. Remuneration rights would be examined and issued much like patents and could be done by existing patent offices. A new agency (or department, for instance, of the Food and Drug Administration (FDA)) would be established to carry out the healthcare impact assessment that would be the basis for awarding funds. A funding body would be established to manage the remuneration rights funds. It should be legally and managerially independent of government but with clear operating parameters.
Institution under remuneration rights Equivalent in the current system
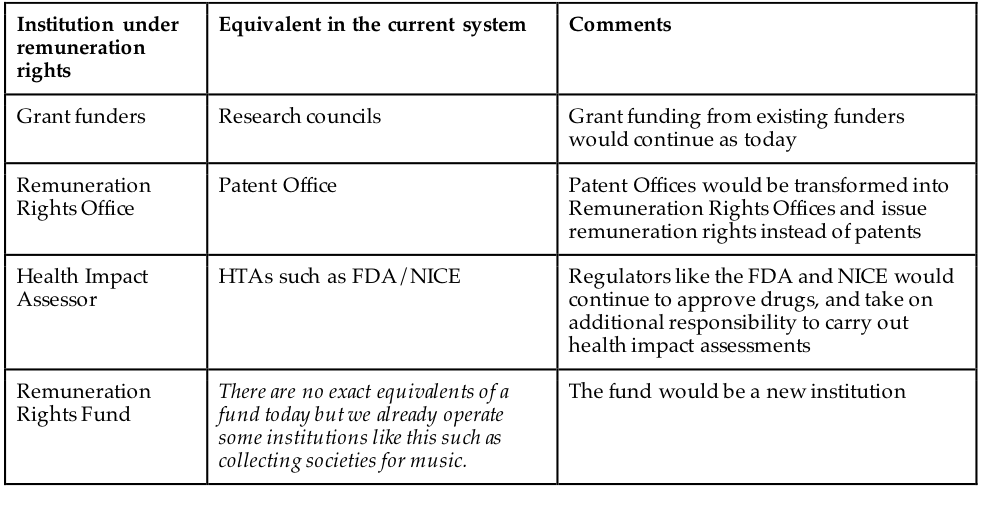
Grant funding from existing funders would continue as today Remuneration Rights Office Patent Office Patent Offices would be transformed into Remuneration Rights Offices and issue remuneration rights instead of patents Health Impact Assessor HTAs such as FDA/NICE Regulators like the FDA and NICE would continue to approve drugs, and take on additional responsibility to carry out health impact assessments Remuneration Rights Fund There are no exact equivalents of a fund today but we already operate some institutions like this such as collecting societies for music. The fund would be a new institution
The changes required to transition to a remuneration rights system could also be rendered less dramatic through a coexistence with patents. Firstly, there is a choice between replacement of prospective patents only, and retrospective replacement. Either way, new medical discoveries would be given remuneration rights, rather than patents, and the question is whether existing patents would be permitted to continue until expiry, or whether these would be converted into remunerations rights with immediate effect. Conversion would have the advantage of simplicity, but the remuneration rights might need to be supplemented by compensation for the sake of fairness.56 If on the other hand only future innovations were to be granted remuneration rights, then patents and remuneration rights would operate alongside one another for a transition period. This could be particularly appropriate as a starting point. Feasibility could be assessed by implementing remuneration rights on a limited basis, which would focus on either a specific area (per country or region) or disease area and operate in parallel of the patent system.
It could also be decided to retain patents permanently in parallel to remuneration rights.57 This would give innovators the choice of filing for either a remuneration right or a patent. In this scenario, the remuneration rights fund would be most attractive for innovations with high demonstrable health impact among low-income populations, where patents offer a weak incentive, and patents would be most attractive for innovations serving wealthier groups with lower health impact. However, this mixed approach would substantially lower the potential benefits and add to complexity so we generally recommend a systemic switch to remuneration rights.
Remuneration rights could also be made more attractive, relative to patents, in a variety of regulatory ways. A large remuneration rights fund would mean the rewards under remuneration rights are high. Government spending on patented drugs could be sharply limited. There could also be direct taxes on income from patents. Full analysis of the implications of these choices over the status of patents under the remuneration rights system must be undertaken in a feasibility study. But the option certainly exists to minimize the transition further by retaining patents temporarily or permanently.
These considerations show that the transition to remuneration rights need not be as dramatic as they first appear. But it is also true that changes of comparable magnitude have been made before without deleterious consequences. The foundation of the National Health Service (NHS) in Britain and of the European Union (EU) and its predecessor alliances are stand-out examples of structural reforms, that occured on a larger scale, that were successful. The establishment of these institutions required profound structural realignment, of a much wider nature than the remuneration rights fund, and on a multinational scale in the case of the EU. They offer reassuring precedents for the implementation of new institutions. There is evidence that the formation of the North American Free Trade Agreement (NAFTA)58 and the entry of the UK into the European Economic Community (EEC)59 did not cause economic disruption, in spite of the scale of these regulatory changes. The remuneration rights model is more limited in its scope than the NHS, NAFTA or the EEC. However, there are parallels of a more comparable scale within the field of pharmaceutical funding mechanisms. The institutionalisation of government research funding in the form of research councils is one such example.60 Such examples demonstrate that it is possible to implement large systematic changes without causing major disruption. Indeed, research by LPL Financial LLC indicates that in the wake of significant events since 1950, the Dow Jones has tended to rise between 3% and 5% after only one month, following initial negative reactions.61 These examples, and typical stock market behaviour in response to major events, suggest that remuneration rights could be introduced seamlessly.
Some general conditions are likely to tend towards successful transitions of this scale. Firstly, it is important that any such transition is consistent with existing international law. Remuneration rights would qualify as a TRIPS flexibility and so meet this condition. Secondly, there must be a sufficient need to justify and motivate a transition on this scale. The problems of access and innovation highlighted in Section 2 amply meet this demand. Thirdly, careful planning is required to avoid shocks to the markets. This would involve extensive work with relevant stakeholders, and capacity building. Such an approach in the case of remuneration rights would particularly involve working with governments, non-governmental organisations and pharmaceutical companies; and building manufacturing capacity. A final criteria likely to tend towards successful large-scale transitions is testing. As already mentioned, remuneration rights could be phased in using pilots or other forms of transition. This would allow lessons to be learned and design to be strengthened, without causing disruption.
A remuneration rights model is superior to patent-driven systems in terms of access, innovation and cost. Remuneration rights offer significant advantages over patents in terms of increasing access and increasing innovation (and its impact), and importantly it does not pit them against each other. Though there would of course be some costs associated with a transition to a remuneration rights model, these would be negligible compared to the benefits of the new system.
The most important criteria for comparing funding mechanisms for pharmaceutical R&D are access, innovation, and cost. These can be defined as follows:
Access. The proportion of potential beneficiaries of a given treatment who actually receive the treatment.
Innovation. The extent to which a given funding mechanism incentivizes innovation based on health impact.
Cost. The operational cost of the system.
Access to medicines increase substantially under a remuneration rights model. In the patent system, the only source of income is through the sale of the final drug, meaning the price of this must reflect both the costs of manufacture, which are low, and R&D costs, which are high. Consequently, only those with sufficient resources can access the drugs produced, and there is a strong incentive to develop marketable, but not necessarily impactful, drugs. There are also few incentives for pharmaceutical companies to distribute their drugs widely beyond the affluent markets that can afford them.
Under remuneration rights, there will be two payment streams, meaning R&D costs are remunerated separately from the sale of drugs. Open information and lack of market exclusivity would encourage competition in manufacture, which would lower drug prices without threatening innovation. Moreover, as remuneration rights are allocated in proportion to health impact rather than market profitability, a new incentive is created to research medicines for conditions mainly affecting the poor. This would increase the number of medicines available, as well as lowering the cost of each individual unit. Companies creating medicines in a remuneration rights based system would also have an incentive to distribute their medicines as widely as possible (provided that the medicine has a positive health impact for the recipients).
The WHO estimates that around a third of the world’s population does not have access to medicines.62 Even assuming only a minority of these people are prevented from accessing medicines because of affordability, decreasing the cost of medicines would still be a health intervention of huge import. Evidence suggests that the generic manufacture remuneration rights enable would lead to significantly reduced prices; when there are 10 or more competitors producing a drug, generic prices approach marginal cost.63 Such costs are likely to be much lower than the prices of branded drugs the patent system requires. Figure 7 illustrates this. The history of antiretroviral drugs, used to combat HIV, also highlight this: under intense international pressure, between 2000 and 2013, prices dropped from \$10,000 per person per year to \$100, a reduction of 99%.64 Given such differences, remuneration rights would significantly improve access when compared with the patent system.
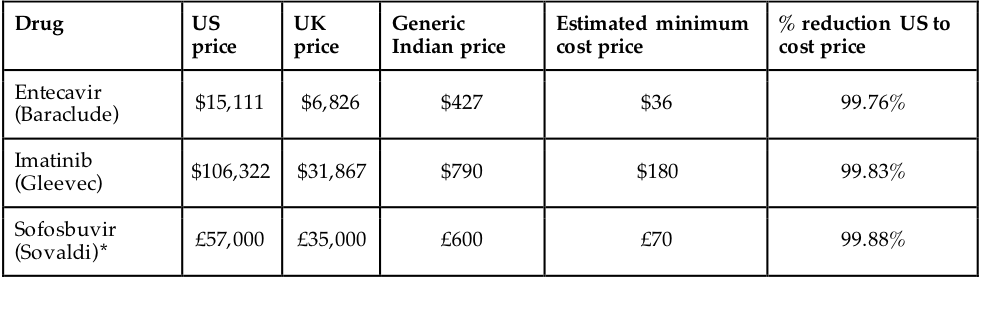
Source: Hill, Andrew. “Generics – the Facts.” presented at the 21st Annual Conference of the British HIV Association (BHIVA), 2015. [http://www.bhiva.org/documents/Conferences/2015Brighton/Presentations/150422/AndrewHill.pdf]{.underline}.
There are important conditions that need to be satisfied in order to lower prices through generic competition. First, only a country or group of countries with multiple pharmaceutical manufacturing firms would be able to generate sufficient competition to lower prices. For a remuneration rights model to be effective, this capacity will need to be already in place, developed prior to launch or be supplemented otherwise.
Second, in some countries most of the supply chain for medicines is owned by pharmaceutical companies. This could potentially restrict generic competition under a remuneration rights system, if wholesalers owned by particular pharmaceutical companies refused to buy from competitors. If participant countries had more open supply chains, this would not be an issue. For cases like the UK and the US, where supply chains are owned by pharmaceutical companies to a large extent, national competitive tendering could resolve this problem.66 Denmark uses such a system today, and drug prices have fallen substantially since 1995, even in the absence of the remuneration rights system.67 Sweden, Germany, the Netherlands and Belgium also operate similar tendering systems for particular sets of drugs.68 While tendering systems require careful design, they tend to lead to reductions in price.69
The most common argument against reducing drug prices is that it will harm future innovation. This argument makes sense in the patent system, where access and innovation are directly traded off against one another. However, the remuneration rights model allows more access while maintaining the same or greater levels of innovation, and does not structure these principles in conflict. The remuneration right itself offers a financial incentive, just like a patent, to undertake research. Provided that the size of the fund were adequate, innovators would receive just as much as under the patent system. Moreover, remuneration rights could also stimulate more effective innovation. Medicines of greater health impact would receive greater remuneration, and those of lesser less. This would direct innovation more effectively, so that for the same amount of money more social value is created and a greater number of people helped. It is also likely that the amount of innovation would increase in a remuneration rights system, as open information permits faster improvements upon the innovations of others.
Patents provide a financial incentive to innovation through market exclusivity, enabling high prices to be charged and costs for R&D recouped through sales. Remuneration rights provide a financial incentive too, in the form of payments from the remuneration rights fund. Provided that this fund is adequately resourced, then incentives for innovation could be maintained at current levels.
Remuneration rights could also incentivize more effective medical innovation. Under the patent system, profits are tied to units sold, not the innovation’s impact. Consequently, when those in need of medicines do not provide a profitable market, for example through poverty or low usage rates, patents fail to incentivize innovation. Given the associations between wealth and health, this is a problematic way of stimulating innovation. First, the poorer you are the more likely you are to be in ill health. Second, diseases are distributed differently between rich and poor populations, meaning that the poor often have different medical needs to the rich. Finally, where cost-effectiveness is concerned, the opportunities for health impact are often especially high in resource poor settings, where general levels of health are poorer and cheaper basic interventions can have a large impact. This compounds the problem. As Scotchmer puts it, “a research agenda driven by patents is hostage to the market and to consumer sovereignty. The consumers who are sovereign are those with resources.”70 Patents stimulate the innovation that wealthy consumers are willing to pay for, and not the innovation that sick consumers need. As Section 2.3 showed, patents are currently failing to incentivize innovation in important disease areas, and over-stimulating less important research. In contrast, remuneration rights incentivize innovation in direct relation to health impact. Profits would be larger for medicines with greater effect, which would be a combination of the severity of the illness treated and the number of patients treated. This means that more effective innovation would be incentivized by remuneration rights than by patents.
Finally, there is also a chance that greater openness in research and innovation would actually increase levels of innovation under the remuneration rights system when compared with patents. Countries such as Italy, Argentina and India for many years did not have patents on drugs, yet they still had very vibrant, innovative and dynamic pharmaceutical industries with substantial innovation in both processes and products. However, with the introduction of patents their industries saw substantial concentration, often coupled with a decline of local industry and the associated innovation.71 Research in other fields suggests similar processes. For instance, the US chemicals industry saw a rapid increase in innovation after the First World War, when German chemical patents were given compulsory licenses, opening information and research to US companies.72 The remuneration rights system could provide a similar boost to innovation while ensuring the innovator is compensated for his work.
Benefits in terms of access and innovation have to be weighed up against the costs of running and transitioning to a remuneration rights system. Transition costs would be considerable. Operational costs would be the same or slightly higher than those associated with the patent system. Comparing these costs with the benefits associated with the remuneration rights system suggests that it would be a better funding mechanism than patents.
Transitioning from the patent system to a remuneration rights model would create large additional costs, albeit for a temporary period. New institutions would have to be established and old ones repurposed; possibly both systems would have to be administered alongside one another for a time. There would be costs to business as new processes were implemented for registering for remuneration rights and for monitoring health impact. A feasibility study should cost such a transition fully - but the total figure would clearly be in billions of dollars.
The transition accomplished, there are then the operational costs of the remuneration rights model to consider. The two most detailed previous proposals to advocate for remuneration rights, MIPF and HIF, provide ballpark figures for the cost of such a fund. MIPF, which would cover all medical innovation in the US, proposed funding levels of 0.55% of GDP annually.73 This would amount to something like \$80 billion.74 HIF, which would be a partial fund operating globally alongside patents, suggested an initial funding level of \$6 billion annually.75 Assuming that HIF represented one third of global product, this would mean a contribution of around 0.03% of GNI annually.76 The suggested costs of HIF and MIPF range widely in part because the funds are designed on different scales, and in part because no rigorous costing analysis has yet been undertaken for remuneration rights models. This would be essential in a feasibility study. At the present time it is clear that billions and probably tens of billions would be required to operate a remuneration rights fund.
Though the remuneration rights model would likely cost billions of dollars to implement, much of this would be offset. The sponsors of the 2011 MIPF bill estimated that the scheme would reduce the cost of drugs in the US by more than \$250 billion.77 This would more than cover the proposed \$80 billion cost to implement the fund. The designers of HIF note a similar phenomenon:
“The net incremental cost to the partner countries would, however, be a fraction of this [0.03% of GNI], since there would be substantial savings from paying low prices on new, patented medicines registered with the HIF… These small net costs are associated with much larger benefits. They would stimulate the development of widely accessible new medicines that greatly reduce morbidity and premature mortality worldwide, would thereby improve global economic performance, and would also reduce dangers from heretofore neglected diseases.“78
Moreover, there is significant deadweight loss associated with the current patent system. Deadweight loss occurs when people who want to pay for a good and can afford to pay more than its marginal cost are unable to do so because they cannot afford the much higher price set.79 In the case of medicines the consumer is often the state, health insurers or hospitals, but the same process applies. Deadweight loss reduces the profits of pharmaceutical companies and the health benefits that would have accrued from wider treatment. Under the remuneration rights system, there would be next to no deadweight loss, as medicines would be sold at close to the production cost. As well as producing savings in terms of the cost of medicines, the introduction of a remuneration rights system would also reduce deadweight losses. Aidan Hollis provides a good summary of the estimates that have been made on pharmaceutical deadweight losses:
“Guell and Fischbaum (1995), using highly aggregated data, claim that the scale of deadweight loss in the US drug market is on the order of \$3bn- \$30bn annually; in a more detailed paper (1997) the same authors estimate deadweight losses of \$5bn on \$8bn of sales, which indicates very large DWL deadweight loss for the market overall. Baker and Chatani (2002) construct a very rough estimate for DWL of \$5bn - \$20bn annually for the US. Globally, the DWL is certain to be many times this figure, because in many markets, drug insurance is unavailable and so consumers are more price-sensitive.“80
In 2004, Baker estimated that annual deadweight loss in the US was around \$25 billion, and would exceed \$100 billion by 2013.81 The vast majority of this loss would be recouped under the remuneration rights system.
Taking into consideration both the transitional and the operational costs of the system, and weighing these against savings in drug prices and reductions in deadweight losses, suggests that the benefits of the remuneration rights system would strongly outweigh the costs. Figure 8 summarises the comparison between remuneration rights and the patent system.
###
| Patents | Remuneration rights | Notes | |
|---|---|---|---|
| Access | ✗✗ | ✓✓ | Remunerations rights permit free market competition in manufacture because research is freey and open for anyone to use. This lowers the price of drugs and provides greater access. Patents use high monopoly prices to recoup the costs of R&D, so fewer people have access to more expensive drugs under this system. In addition, lower prices and increased access especially helps the poor. Those with fewer resources lose out disproportionately from high drug prices under patents and gain disproportionately from lower prices under remuneration rights. Additionally, under patents there are limited incentives to innovate for diseases of the poor, because they do not present a lucrative potential market. There is consequently a dearth of R&D to begin with for diseases of the poor, which reduces the medicines available for them. Under remuneration rights, by default, all health impact is treated equally whether a rich or a poor group benefits. |
| Innovation | ✓ | ✓✓ | Remuneration rights and patents provide the same level of financial incentive to innovators. Whilst similar to patents in this general respect, remuneration rights may also provide advantages over patents regarding innovation: - Payments to innovators are directly linked to health impact under remuneration rights, which means that innovation incentives are more directly tied to outcomes and value than under patents. - Remuneration rights allow open access to all research, which stimulates follow-on innovation and reduces the risk of holdup. |
| Cost | ✓ | ✓ | The costs of operating the remuneration rights system would be similar to that of running the current system we have. The patent office would become a remuneration office, grants would continue as today, health impact assessment would be performed by HTAs that already exist etc. The only additional expense would be the actual administration of the remuneration rights fund but this should be relatively limited given the transparent, pre-defined nature of the distributions (and this may even be cost-saving if it saves on current bureaucracy elsewhere in the system). |
We urgently need to reconceptualize how we think of and finance medical innovation. The patent monopoly system positions access to medicines and innovation as inevitably in tension. Millions globally cannot access the medicines they need to survive and thrive, and many areas urgently requiring new medicines are neglected due to the lack of a profitable market. Such a system fails society, and it also fails industry, by obstructing research and slowly but inevitably changing public perception of pharmaceutical companies from contributors to the public good to fraudulent exploiters of the vulnerable.
By rewarding innovation and manufacture separately, we can resolve this structural opposition and have a win-win: access and innovation at the same time. Moreover, by rewarding innovation based on health impact rather than final sales, we can refocus the aims of the pharmaceutical industry on promoting health – whilst maintaining profits. This is a boon to society and to industry, vastly expanding both access and the avenues of possible research that might be pursued, not to mention the speed at which this might be done.
This change is both desirable and possible. The structural and infrastructural precursors for a remuneration rights based system are already in place, and many of them can be drawn from existing intellectual property and public health systems. Beyond being practically feasible, such a change is also politically desirable, and importantly in a bipartisan way. Expanded access to healthcare benefits all, and enhanced avenues for innovation and remuneration are a boon to small and big businesses alike.
Now is the time for change. The current patent-driven mechanism of medical innovation is not working for anyone. This paper puts forth the case for implementing a large systemic change, showing this not only to be necessary but also incredibly desirable and feasible. It is important that this theoretical work is supplemented by a rigorous, empirical feasibility study, demonstrating how such a system would work in reality. Given the significant systemic failures of the current system, and the amounts of money spent attempting to balance access and innovation, there is clear momentum to do so.
Ahead of us is the possibility of a world where medical innovation is cutting edge, profitable, impactful and available to all. No one should die for lack of access to a medicine that exists and has been paid for yet is held out of reach, nor should researchers and industry have to choose between developing genuinely useful therapies or those of little impact that generate profit. A better system of improved access and innovation is achievable, and given the manifest problems today a priority of public health research should be exploring this possibility.
=
“2015 Patent Dispute Report.” Unified Patents. Accessed December 22, 2017. [https://www.unifiedpatents.com/news/2016/5/30/2015-patent-dispute-report]{.underline}.
“A Look Back at Pharmaceuticals in 2006: Aggressive Advertising Cannot Hide the Absence of Therapeutic Advances.” Prescrire International 16, no. 88 (2006): 80–86.
Angell, Marcia. The Truth about the Drug Companies: How They Deceive Us and What to Do about It. New York: Random House, 2004.
“Anti-Aging Market (Baby Boomer, Generation X and Generation Y), by Product (Botox, Anti-Wrinkle Products, Anti-Stretch Mark Products, and Others), by Services (Anti-Pigmentation Therapy, Anti-Adult Acne Therapy, Breast Augmentation, Liposuction, Chemical Peel, Hair Restoration Treatment, and Others), by Device (Microdermabrasion, Laser Aesthetics, Anti-Cellulite Treatment and Anti-Aging Radio Frequency Devices) : Global Industry Perspective, Comprehensive Analysis, Size, Share, Growth, Segment, Trends and Forecast, 2015 – 2021.” Market Report. Zion Market Research, 2016. [https://www.zionmarketresearch.com/toc/anti-aging-market]{.underline}.
Baker, Dean. “Financing Drug Research: What Are the Issues?” Washington, DC: Issue Brief, Center for Economic and Policy Research, 2004.
Barral, P. Étienne, and Rhône-Poulenc Rorer Foundation. 20 Years of Pharmaceutical Research Results throughout the World (1975-94). Antony, France: Rhône-Poulenc Rorer Foundation, 1996.
Bigdeli, Maryam, David H. Peters, and Anita K. Wagner. “Medicines in Health Systems: Advancing Access, Affordability and Appropriate Use,” 2014. [http://apps.who.int/iris/bitstream/10665/179197/1/9789241507622_eng.pdf]{.underline}.
Boldrin, Michele, and David K. Levine. Against Intellectual Monopoly. Ebook Central. New York: Cambridge University Press, 2008. [https://ezproxy-prd.bodleian.ox.ac.uk/login?url=http://ebookcentral.proquest.com/lib/oxford/detail.action?docID=352991]{.underline}.
Burfisher, Mary E., Sherman Robinson, and Karen Thierfelder. “The Impact of NAFTA on the United States.” Journal of Economic Perspectives 15, no. 1 (2001): 125–144. [https://doi.org/10.1257/jep.15.1.125]{.underline}.
Burt, James. “Lessons to Be Learnt? - An Overview of Tendering Processes for Medicines across Europe.” Accord, n.d. [http://www.accord-healthcare.eu/files/files/homepage-files/Tendering%20system%20BJMP%20by%20James%20Burt.pdf]{.underline}.
Crafts, Nicholas. “The Growth Effects of EU Membership for the UK: Review of the Evidence.” Global Perspectives Series, 2016. [http://www.smf.co.uk/wp-content/uploads/2016/04/SMF-CAGE-The-Growth-Effects-of-EU-Membership-for-the-UK-a-Review-of-the-Evidence-.pdf]{.underline}.
Crow, David. “Pfizer Raises US Prices of 91 Drugs by 20% in 2017.” Financial Times, June 2, 2017. [https://www.ft.com/content/b2e0dd80-47ab-11e7-8519-9f94ee97d996]{.underline}.
Cunningham, Paul, Abdullah Gök, and Philippe Laredo. “The Impact of Direct Support to R&D and Innovation in Firms.” Nesta Working Paper No. 13⁄03, 2013. [https://www.nesta.org.uk/sites/default/files/the_impact_of_direct_support_to_rd_and_innovation_in_firms.pdf]{.underline}.
“Delivering on the Global Partnership for Achieving the Millennium Development Goals.” United Nations, MDG Gap Task Force, 2008. [http://www.who.int/medicines/mdg/MDG08ChapterEMedsEn.pdf]{.underline}.
Drummond, Michael. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford Medical Publications. Oxford: Oxford University Press, 2005.
Dylst, Pieter, Arnold Vulto, and Steven Simoens. “Tendering for Outpatient Prescription Pharmaceuticals: What Can Be Learned from Current Practices in Europe?” Health Policy 101, no. 2 (July 1, 2011): 146–52. [https://doi.org/10.1016/j.healthpol.2011.03.004]{.underline}.
Frost, Laura J., Michael R. Reich, and others. Access: How Do Good Health Technologies Get to Poor People in Poor Countries? Harvard Center for Population and Development Studies, 2008. [https://www.cabdirect.org/cabdirect/abstract/20103004633]{.underline}.
“Fund for Research and Development in Neglected Diseases.” CEWG submission, 2011. [http://www.who.int/phi/news/phi_20_cewg_frind_en.pdf]{.underline}.
“Geneva Technical Workshop on Proposals for a Cancer Innovation Fund (CIF) – Union for Affordable Cancer Treatment,” 2017. [https://cancerunion.org/2017/02/01/101/]{.underline}.
“GHO | By Category | Antiretroviral Therapy Coverage - Data and Estimates by Country.” WHO. Accessed October 25, 2017. [http://apps.who.int/gho/data/node.main.626]{.underline}.
“GHO | By Category | Treatment Coverage - Data by WHO Region.” WHO. Accessed October 31, 2017. [http://apps.who.int/gho/data/view.main.57056ALL?lang=en]{.underline}.
“Global Hepatitis Report, 2017.” World Health Organisation, 2017. [http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1]{.underline}.
“Global Strategy on Public Health, Innovation and Intellectual Property.” WHA, 2008. [http://apps.who.int/gb/ebwha/pdf_files/WHA61-REC1/A61_Rec1-part2-en.pdf?ua=1]{.underline}.
Gold, Marthe R. Cost-Effectiveness in Health and Medicine. Ebook Central. New York: Oxford University Press, 1996. [https://ezproxy-prd.bodleian.ox.ac.uk/login?url=http://ebookcentral.proquest.com/lib/oxford/detail.action?docID=679610]{.underline}.
“Health Spending Explorer.” Peterson-Kaiser Health System Tracker (blog). Accessed October 25, 2017. [https://www.healthsystemtracker.org/interactive/]{.underline}.
Hill, Andrew. “Generics – the Facts.” presented at the 21st Annual Conference of the British HIV Association (BHIVA), 2015. [http://www.bhiva.org/documents/Conferences/2015Brighton/Presentations/150422/AndrewHill.pdf]{.underline}.
“Historical Stock Market Reactions to Geopolitical and Economic Crisis Events.” LPL Financial Research (blog), April 21, 2017. [https://lplresearch.com/2017/04/21/historical-stock-market-reactions-to-geopolitical-and-economic-crisis-events/]{.underline}.
Hollis, Aidan. “An Efficient Reward System for Pharmaceutical Innovation,” 2005. [http://www.who.int/intellectualproperty/news/en/Submission-Hollis6-Oct.pdf]{.underline}.
Hollis, Aidan, and Thomas Pogge. The Health Impact Fund: Making New Medicines Accessible for All. Incentives for Global Health, 2008. [http://healthimpactfund.org/wp-content/uploads/2015/12/hif_book.pdf]{.underline}.
“Horizon Scan Report.” iMed: Innovating Medical Entrepreneurship and Delivery, 2017. [http://imedproject.org/research/horizon-scan/imed-horizon-scan-report.pdf]{.underline}.
“How a Global R&D Convention Could Fill the Gaps Left by Today’s Medical Innovation System.” MSF, 2012. [https://www.msfaccess.org/sites/default/files/MSF_assets/Innovation/Docs/MedInno_Briefing_GlobalConventionRD_ENG_2012Update.pdf]{.underline}.
Hunt, M.I. “Prescription Drugs and Intellectual Property Protection: Finding the Right Balance between Access and Innovation.” Washington, DC: NIH, 2000.
Jon Cohen, Jon. “How the Battle Lines over CRISPR Were Drawn.” Science | AAAS, February 15, 2017. [http://www.sciencemag.org/news/2017/02/how-battle-lines-over-crispr-were-drawn]{.underline}.
“Judging Whether Public Health Interventions Offer Value for Money | Guidance and Guidelines.” Accessed November 29, 2017. [https://www.nice.org.uk/advice/lgb10/chapter/judging-the-cost-effectiveness-of-public-health-activities]{.underline}.
Kaitin, K. I., N. R. Phelan, D. Raiford, and B. Morris. “Therapeutic Ratings and End-of-Phase II Conferences: Initiatives to Accelerate the Availability of Important New Drugs.” Journal of Clinical Pharmacology 31, no. 1 (January 1991): 17–24.
Kamal, Babah, Cox, Cynthia. “What Are the Recent and Forecasted Trends in Prescription Drug Spending?” Peterson-Kaiser Health System Tracker (blog). Accessed November 16, 2017. [https://www.healthsystemtracker.org/chart-collection/recent-forecasted-trends-prescription-drug-spending/]{.underline}.
Kanavos, Panos, Elizabeth Seeley, and Sotiris Vandoros. “Tender Systems for Outpatient Pharmaceuticals in the European Union: Evidence from the Netherlands, Germany and Belgium.” Enterprise and Industry, European Commission, 2010.
Koskinen, Hanna, Elina Ahola, Leena K Saastamoinen, Hennamari Mikkola, and Jaana E Martikainen. “The Impact of Reference Pricing and Extension of Generic Substitution on the Daily Cost of Antipsychotic Medication in Finland.” Health Economics Review 4 (August 19, 2014). [https://doi.org/10.1186/s13561-014-0009-3]{.underline}.
Kremer, Michael R. “Patent Buyouts: A Mechanism for Encouraging Innovation” 113, no. 4 (1998): 1137–67. [https://doi.org/10.1162⁄003355398555865]{.underline}.
Lesher, Molly, and Sébastien Miroudot. “Analysis of the Economic Impact of Investment Provisions in Regional Trade Agreements.” IDEAS Working Paper Series from RePEc, 2006.
Light, Donald W., and Joel R. Lexchin. “Pharmaceutical Research and Development: What Do We Get for All That Money?” BMJ 345 (2012): e4348. [https://doi.org/10.1136/bmj.e4348]{.underline}.
“Longitude Prize | Enter the Prize.” Accessed August 16, 2017. [https://longitudeprize.org/]{.underline}.
Love, James. “Measures to Enhance Access to Medical Technologies, and New Methods of Stimulating Medical R&D.” U.C. Davis Law Review 40 (2007 2006): 679.
Love, James, and Tim Hubbard. “Prizes for Innovation of New Medicines and Vaccines.” Annals of Health Law 18, no. 2 (2009): 155–86, 8 p. preceding i.
———. “The Big Idea: Prizes to Stimulate R&D for New Medicines.” Chicago-Kent Law Review 82 (2007): 1519.
Matthijs, Gert, and Dicky Halley. “European-Wide Opposition against the Breast Cancer Gene Patents.” European Journal of Human Genetics 10, no. 12 (December 2002): 783. [https://doi.org/10.1038/sj.ejhg.5200924]{.underline}.
“Medicines Patent Pool.” Accessed July 13, 2017. [http://www.medicinespatentpool.org]{.underline}.
Morgan, Steven G., Kenneth L. Bassett, James M. Wright, Robert G. Evans, Morris L. Barer, Patricia A. Caetano, and Charlyn D. Black. “‘Breakthrough’ Drugs and Growth in Expenditure on Prescription Drugs in Canada.” BMJ (Clinical Research Ed.) 331, no. 7520 (2005): 815–16.
Moser, Petra, and Alessandra Voena. “Compulsory Licensing: Evidence from the Trading with the Enemy Act.” American Economic Review 102, no. 1 (2012): 396–427. [https://doi.org/10.1257/aer.102.1.396]{.underline}.
Motola, Domenico, Fabrizio De Ponti, Elisabetta Poluzzi, Nello Martini, Pasqualino Rossi, Maria Chiara Silvani, Alberto Vaccheri, and Nicola Montanaro. “An Update on the First Decade of the European Centralized Procedure: How Many Innovative Drugs?” British Journal of Clinical Pharmacology 62, no. 5 (2006): 610–616. [https://doi.org/10.1111/j.1365-2125.2006.02700.x]{.underline}.
Neumann, Peter J. Using Cost-Effectiveness Analysis to Improve Health Care: Opportunities and Barriers. Oxford Scholarship Online. New York ; Oxford: Oxford University Press, 2005. [https://ezproxy-prd.bodleian.ox.ac.uk/login?url=http://dx.doi.org/10.1093/acprof:oso/9780195171860.001.0001]{.underline}.
“New Products and New Indications in 2016: A System That Favours Imitation over the Pursuit of Real Progress.” Prescrire International 26, no. 182 (2017): 136–39.
Pedersen, K. Møller. “Pricing and Reimbursement of Drugs in Denmark.” The European Journal of Health Economics 4, no. 1 (March 1, 2003): 60–65. [https://doi.org/10.1007/s10198-003-0165-6]{.underline}.
Perlberg, Steven. “This Is What Happens To The Stock Market When Terrible Things Happen In The World.” Business Insider. Accessed November 29, 2017. [http://www.businessinsider.com/how-stocks-react-geopolitical-events-2014-3]{.underline}.
Peters, David H., Anu Garg, Gerry Bloom, Damian G. Walker, William R. Brieger, and M. Hafizur Rahman. “Poverty and Access to Health Care in Developing Countries.” Annals of the New York Academy of Sciences 1136, no. 1 (2008): 161–71. [https://doi.org/10.1196/annals.1425.011]{.underline}.
“Pharma 2020: The Vision Which Path Will You Take?” Price Waterhouse Coopers, n.d. [https://www.pwc.com/gx/en/pharma-life-sciences/pdf/pharma2020final.pdf]{.underline}.
“Promoting Innovation and Access to Health Technologies.” Report of the UNITED NATIONS SECRETARY-GENERAL’S HIGH-LEVEL PANEL ON ACCESS TO MEDICINES, 2016. [https://static1.squarespace.com/static/562094dee4b0d00c1a3ef761/t/596fed6d914e6b24d15ece26/1500507506991/50923+-+HLP+Report+-+ENGLISH+-+web_v3.pdf]{.underline}.
Ravvin, Michael. “Incentivizing Access and Innovation for Essential Medicines: A Survey of the Problem and Proposed Solutions.” Public Health Ethics 1, no. 2 (2008): 110–23. [https://doi.org/10.1093/phe/phn017]{.underline}.
Reiffen, David, and Michael R. Ward. “Generic Drug Industry Dynamics.” Review of Economics and Statistics 87, no. 1 (2005): 37–49. [https://doi.org/10.1162⁄0034653053327694]{.underline}.
“Research and Development to Meet Health Needs in Developing Countries: Strengthening Global Financing and Coordination.” Report of the Consultative Expert Working Group on Research and Development: Financing and Coordination. World Health Organisation, 2012. [http://apps.who.int/iris/bitstream/10665/254706/1/9789241503457-eng.pdf?ua=1]{.underline}.
“Resolution On Cancer Hailed By WHO Members, Easily Adopted In Committee.” Intellectual Property Watch (blog), 2017. [https://www.ip-watch.org/2017/05/31/resolution-cancer-hailed-members-easily-adopted-committee/]{.underline}.
Røttingen, John-Arne, Sadie Regmi, Mari Eide, Alison J. Young, Roderik F. Viergever, Christine Årdal, Javier Guzman, Danny Edwards, Stephen A. Matlin, and Robert F. Terry. “Mapping of Available Health Research and Development Data: What’s There, What’s Missing, and What Role Is There for a Global Observatory?” The Lancet 382, no. 9900 (2013): 1286–1307. [https://doi.org/10.1016/S0140-6736(13)61046-6]{.underline}.
Sanders, Bernard. “Text - H.R.417 - 109th Congress (2005-2006): Medical Innovation Prize Act of 2005.” Webpage, 2005. [https://www.congress.gov/bill/109th-congress/house-bill/417/text]{.underline}.
———. “Text - S.495 - 115th Congress (2017-2018): Medical Innovation Prize Fund Act.” Webpage, 2017. [https://www.congress.gov/bill/115th-congress/senate-bill/495/text]{.underline}.
———. “Text - S.627 - 113th Congress (2013-2014): Medical Innovation Prize Fund Act.” Webpage, 2013. [https://www.congress.gov/bill/113th-congress/senate-bill/627/text]{.underline}.
———. “Text - S.1137 - 112th Congress (2011-2012): Medical Innovation Prize Fund Act.” Webpage, 2012. [https://www.congress.gov/bill/112th-congress/senate-bill/1137/text]{.underline}.
———. “Text - S.1138 - 112th Congress (2011-2012): Prize Fund for HIV/AIDS Act.” Webpage, 2012. [https://www.congress.gov/bill/112th-congress/senate-bill/1138/text]{.underline}.
———. “Text - S.2210 - 110th Congress (2007-2008): Medical Innovation Prize Act of 2007.” Webpage, 2007. [https://www.congress.gov/bill/110th-congress/senate-bill/2210/text]{.underline}.
Scotchmer, Suzanne. Innovation and Incentives. Cambridge, Mass. ; London: MIT Press, 2004.
Seddoh, Anthony, Adiele Onyeze, John Owusu Gyapong, Janet Holt, and Donald Bundy. “Towards an Investment Case for Neglected Tropical Diseases.” LANCET COMMISSION ON INVESTING IN HEALTH WORKING PAPER, 2013. [http://globalhealth2035.org/sites/default/files/working-papers/towards-an-investment-case.pdf]{.underline}.
“Selected Innovation Prizes and Reward Programs.” KEI Research Note. Knowledge Ecology International, 2008. [https://www.keionline.org/misc-docs/research_notes/kei_rn_2008_1.pdf]{.underline}.
Smedley, Tim. “Is It Fair to Accuse the Pharma Industry of Neglecting Tropical Diseases?” The Guardian, October 15, 2015, sec. Guardian Sustainable Business. [http://www.theguardian.com/sustainable-business/2015/oct/15/pharma-industry-neglecting-tropical-diseases-snake-bite]{.underline}.
Stiglitz, Joseph E. “Economic Foundations of Intellectual Property Rights.” Duke Law Journal 57, no. 6 (2008): 1693–1724. [https://doi.org/10.2307⁄40040630]{.underline}.
“The Medical Innovation Prize Fund: A New Paradigm for Supporting Sustainable Innovation and Access to New Drugs: De-Linking Markets for Products from Markets for Innovation,” 2011. [https://www.keionline.org/sites/default/files/big_prize_fund_overview_26may2011_letter.pdf]{.underline}.
“The PDP+ Fund: Accelerating R&D in New Products for Diseases of Poverty.” Science Speaks: HIV & TB News (blog), 2010. [https://sciencespeaks.wordpress.com/2010/05/17/the-pdp-fund-accelerating-rd-in-new-products-for-diseases-of-poverty/]{.underline}.
“The Swedish Pharmaceutical Reimbursement System.” LFN Pharmaceutical Benefits Board, 2007. [https://www.tlv.se/upload/english/eng-swe-pharma-reimbursement-system.pdf]{.underline}.
“The World Bank | Data, United-States.” The World Bank | Data, 2017. [https://data.worldbank.org/country/united-states]{.underline}.
“Untangling the Web of Antiretroviral Price Reductions.” MSF, 2013. [https://www.msf.org/sites/msf.org/files/msf_access_utw_16th_edition_2013.pdf]{.underline}.
Van Luijn, Johan C. F., Frank W. J. Gribnau, and Hubert G. M. Leufkens. “Superior Efficacy of New Medicines.” European Journal of Clinical Pharmacology 66, no. 5 (2010): 445–448. [https://doi.org/10.1007/s00228-010-0808-3]{.underline}.
“WHA59.24: Public Health, Innovation, Essential Health Research and Intellectual Property Rights: Towards a Global Strategy and Plan of Action,” 2006. [http://trade.ec.europa.eu/doclib/docs/2006/october/tradoc_130884.pdf]{.underline}.
“WHO | Estimates of Disease Burden and Cost-Effectiveness.” WHO. Accessed September 11, 2017. [http://www.who.int/immunization/monitoring_surveillance/burden/estimates/en/]{.underline}.
“WHO | Hepatitis C.” WHO. Accessed October 25, 2017. [http://www.who.int/mediacentre/factsheets/fs164/en/]{.underline}.
“WHO | Immunization Coverage.” WHO. Accessed September 11, 2017. [http://www.who.int/mediacentre/factsheets/fs378/en/]{.underline}.
“WHO | Meeting the Need for Treatment: The Initiatives.” WHO. Accessed June 26, 2017. [http://www.who.int/bulletin/volumes/84/5/news30506/en/]{.underline}.
Wirtz, Veronika J., Hans V. Hogerzeil, Andrew L. Gray, Maryam Bigdeli, Cornelis P. de Joncheere, Margaret A. Ewen, Martha Gyansa-Lutterodt, et al. “Essential Medicines for Universal Health Coverage.” The Lancet 389, no. 10067 (2017): 403–76. [https://doi.org/10.1016/S0140-6736(16)31599-9]{.underline}.
Figure 1: US prescription drug expenditure Figure 2: Trends in therapeutic benefit Figure 3: Access to treatment for HIV, Hepatitis C and TB Figure 4: The remuneration rights model Figure 5: The flow of money and medicines under the remuneration rights system Figure 6: Common institutions in the remuneration rights and patent systems Figure 7: Difference between branded and generic prices Figure 8: Summary of comparison between remuneration rights and patents
DALY Disability-Adjusted Life Years DWL Deadweight Loss EEC European Economic Area EU European Union FDA Food and Drug Administration HIF Health Impact Fund HIV Human Immunodeficiency Virus MIPF Medical Innovation Prize Fund NAFT North American Free Trade Agreement NHS National Health Service NICE National Institute for Health and Care Excellence QALY Quality-Adjusted Life Years R&D Research and Development TB Tuberculosis TRIPS Agreement on Trade-Related Aspects of Intellectual Property Rights UK United Kingdom US United States WHO World Health Organization
Access To Medicines (ATM) ‘is defined as having medicines continuously available and affordable at public or private health facilities or medicine outlets that are within one hour’s walk from the homes of the population\‘.82 A pared down way of defining access is the proportion of the potential beneficiaries of a given treatment who actually receive the treatment.
Deadweight loss is a loss of economic efficiency. The phrase often describes circumstances where consumers want to buy what a producer wants to sell, but this fails to happen because of a market inefficiency, often with regards pricing. Scotchmer writes, \‘Deadweight loss occurs when people are excluded from using the good even though their willingness to pay are higher than the marginal cost.\’83 This is a particular problem in monopoly settings, were lack of competition means that the price can be set at a high level, thus excluding those with lower willingness to pay. It also becomes an especially stark problem in the case of information goods, where the marginal cost is very low or even nothing (and thus the most efficient distribution would be to grant universal access).
A health impact can be positive or negative. A positive health impact is an effect which contributes to good health or to improving health. A negative health impact has the opposite effect, causing or contributing to ill health84. Within the context of remuneration right model, assessing positive health impact is central to the allocation of \“equitable remuneration\” from the remuneration right fund. We propose to measure health impact using QALYs and DALYs.
Marginal cost means the additional cost of producing one more unit of a good or service. It is different from the average cost, which includes fixed costs as well as variable costs. Marginal costs only include the variable costs (once the factory has been built in the first place, there is no additional fixed cost to producing one more rubber toy). In the case of information goods, marginal costs are low and fixed costs high.
A system where innovators are awarded repeated remuneration rights after registering their innovation. Remuneration rights would give their owners the right to payment from a central fund according to the value generated by the innovation: how much a given drug improved health.
Cancer Innovation Fund (CIF)86 A proposal to delink R&D costs from drug and vaccine prices in the case of cancer. Proposed by various actors in 2008, 2009, 2014 and 2017. The proposal is broad in allowing for any kind of delinkage of R&D and manufacture and does not currently detail a specific favoured alternative. Currently under discussion. Medical Innovation Prize Fund (MIPF)87 A proposal for a compulsory fund to replace the patent monopoly rights system in the US and remunerate innovators on the basis of the health impact they create. Presented by Bernie Sanders to the House of Representatives in 2005 and the Senate in 2007, 2011, 2013 and 2017. Currently under discussion. Health Impact Fund (HIF)88 A proposal to create an optional fund which would remunerate medical R&D according to health impact. Proposed by Hollis and Pogge in 2008. Discussed largely in academic circles. Global framework for essential health R&D89 A series of proposals to the WHO to create a global framework committing states to contribute a certain level of funding into a pooled fund, which would be disbursed in a variety of ways including remuneration rights. The ‘global framework’ in particular refers to a 2006 resolution, but here shall be used as an umbrella term for the process relating to such proposals which began in 2005, included the 2008 global strategy and plan of action on medical R&D funding and coordination and culminated in 2012. A process which still has repercussions today but whose vision has not been fully realised. There is still no global pooled fund. PDP+ Fund90 A proposal for a fund for R&D into neglected diseases, which awards remuneration to innovators on condition of pro-access measures. Proposed by Novartis, the George Institute, and [IAVI]{.underline} in 2010. A one-off proposal. Fund for Research and Development in Neglected Diseases (FRIND)91 A proposal for an optional fund for R&D into neglected diseases which awards remuneration to innovators provided that their drugs are sold affordably. Proposed by Novartis to the EWG in 2009 and the [CEWG]{.underline} in 2011. A one-off proposal. Australian Democrats Prize Proposal92 An optional, international public good patent scheme, where innovators are rewarded from a fund in relation to health impact. Proposed in 2007 by the Australian Democrats. A one-off proposal.
Prizes as a funding mechanism boast many successfully implemented proposals. Prizes take many forms, including milestone prizes, end prizes, tournaments and advanced market commitments. In terms of access and innovation, there is a disjunct between the technically possible and the actually implemented where it comes to prizes. Prizes can be made conditional on access provisions, and there is no reason that a prize should not be made conditional on health impact and thus provide an efficient incentive. However, while prizes often come with access requirements, they are not usually set in relation to health impact because of the difficulty of measuring these same. Instead, prizes tend to focus on a specified research achievement. This means they do not necessarily incentivize innovation in relation to health impact, and so are only a partial solution to the access and innovation problem.
Grants are a push funding mechanism: grants fund research upfront, rather than after the fact.93 Currently, grants are a very widely used form of funding in medical R&D, and are uncontroversial. Even strong opponents of the current system agree that grants should continue to operate as a funding mechanism.94 Grants are the most suitable mechanisms for early stage research, as information remains open for others to build upon and funding is not tied to specific outcomes, allowing exploratory work. Among the many proposals to improve the current state of medical R&D, few proposals concern improving the grants system, which also suggests that this funding mechanism is working reasonably.95 However, as a funding mechanism, grants have little to do with access. They provide no incentive to translate research into a marketable product, so this stage in development is usually undertaken by commercial firms who then patent the results.96 This makes grant funding poorly suited to dealing with the problem of access. Grant funding does provide a significant boost to innovation, but does not exert strong incentives regarding health impact. This is because grants are provided upfront, and it is very difficult to predict health impact before the fact. It might also be undesirable for all funding to be directly tied to health impact: we need basic research, and grant funding is excellent at resourcing this. Essential though grant funding will remain, its functioning is only distantly related to the problems of access and impact-based innovation.
An SCQH is a problem solving tool. It can be used in a number of ways, here it is particularly useful to structure our research. SCQH stands for Situation, Complication, Question and Hypothesis. The goal is to pave the way toward a clearly formulated Hypothesis, which can then be tested.
Medicines are expensive to research and cheap to make and millions of people need them; meanwhile funding mechanisms are not directly linked to health impact, profits are based on prices, and the existence of monopoly patents supports prices well above the cost of manufacture.
Monopoly patents fund innovation through high prices, creating an inevitable tension between access and innovation; and currently denying access to medicines for millions of people through inflated prices and lack of innovation in non-profitable areas, and failing to incentivize for health impact or efficiency of research and manufacture.
What funding mechanisms can replace the tension between innovation and access inherent in the current patent system; incentivising innovation based on cost effective health impact, providing incentives for innovation as high as today, and providing access at close to the cost of manufacture.
The best resolution to the tension between access and innovation is a remuneration rights model that removes the dilemma and offers incentives for both innovation and access; it provides a free market, state-independent mechanism resourced by the state and philanthropists that incentivizes innovations via remuneration based on health impact, on condition that the innovations are free to use and unrestricted, allowing for competition in manufacturing and therefore lower prices for medicines whilst providing incentives for innovators at a similar level to today.
At the most basic level, health impact can be measured simply by dividing the cost of treatment by the number of lives saved.97 However, with this approach it is hard to compare between interventions: is \$100 for a successful hip replacement more or less effective than \$100 for a cancer diagnosis? It is therefore necessary to standardise when measuring health impact.98 The most common form of standardisation is the QALY.99 This metric assigns a year of perfect health the value 1, and death the value 0. All states of health short of perfect health can then be measured on this scale.100 An alternative metric, more common in global health,101 is the DALY. This also adjusts the value of a year of life, but using the severity of particular disabling health outcome as a weighting.102
These measurements constitute a robust base which could be elaborated upon. Each scale could be adjusted further to ensure appropriate and fair incentive is provided to reflect the medical landscape as a whole. For example, in the case orphan diseases, a logarithmic weighting could provide these scales with the nuance required to incentivize their research and innovation as well as for blockbuster drugs. Different weighting methods would have to be compared to the the simple linear proportional QALY payment to ensure all illnesses are considered and incentivized appropriately. Alternatively, special values could also be created to fit the incentive required for orphan disease innovation. Finally, there are alternative measurement systems, such as Healthy Year Equivalents or Saved-Young-Life Equivalents,103 all of which could be explored in a feasibility study for a remuneration rights fund.
If the remuneration rights model is desirable, practical, economically fit, legal and possible, it is fair to wonder whether similar initiatives have been done before. Spotify, the music streaming service, is a good example of a business that has exploited - to an extent - the model which reward innovators without restricting access. The platform opens a large music library to its users for a monthly cost, and redistributes the bulk of their income to the artists in proportion of their impact (calculated according to a number of metrics including artist popularity and number of streams). With over 140 million users, Spotify is one of the most popular means to access music globally.
Much like medicines, music has extremely low cost of copy (close to zero), with larger costs of production combined with millions of people willing to stream and listen. But the traditional way to buy music, through highly priced individual items (tracks or albums) in exchange of lifelong access, greatly limits the breadth of music one can access. Paying per item is not only an inefficient investment of user’s money, it also reduces user’s capacity to discover new artists and allocate their funds more evenly. That’s why Spotify’s monthly fee - currently \$9.99 - for an unlimited access to Spotify’s entire music catalogue is very competitive and the company fast growing. Between March and July 2017, the company boasted the addition of 10 million paying subscribers.
Spotify operates in many similar ways to the proposed Remuneration Rights model. The provider gathers a large music library on its platform and users pay a monthly subscription to access the library. The money is then centralized by Spotify, much like the Remuneration Rights would pool its fund together by gathering health buyers contributions. Spotify then redistributes 70% of its income to compensate the content creators who drive the platform - or more accurately to their record label. The level of compensation is proportional to the impact of an artists music. This is very similar to how pharmaceutical innovators would be remunerated under a Remuneration Rights model, which would be determined as a function of the health impact of their drug. As a result, people have access and freedom to explore a broad range of music for a fixed price while ensuring creators are still rewarded for their efforts. This is similar to how people will be able to access the medicines they need for an affordable price while still allowing innovators to be rewarded.
This is technically feasible because, like innovators, artists are clearly identified as the authors of their work. The platform has a built in system that quantifies the ‘impact’ of each song by recording how many times it has been played. The political management is slightly different than what is commonly assumed. Artists do not receive a fixed amount of money per stream. Instead, the total royalties (70%) are split and distributed depending on what percentage of total Spotify streams a song has garnered. As a result, remuneration fluctuates each month depending on the number of subscribers paying into the system and what music is played that month. Additionally, the money does not go directly go to the artist. Record labels often license the music to Spotify, then distribute the remuneration they receive amongst their artists after taking a cut, the size of which will typically depend on individual record contracts. While Spotify’s model is not perfect or optimal (its library lacks in openness, for example, and there have been some criticisms of unfair remuneration by some high profile artists), but it is operational, legal and successful.
Spotify proves that these systems can and do work, allowing access and innovation to thrive together. Like the Remuneration Rights model, the overall improvements in terms of increased access and reduced cost, by far outweighs the limitations of the Spotify model. While Spotify is limited in its openness (its library is not complete) and its prices are higher than cost, due to the private holding of the company. Such pitfalls would not occur within the Remuneration Rights system. First, because open and universal access - to innovative knowledge and medicines - are a fundamental priority of the model, the restrictions of Spotify’s limited catalogue will be overcome. Second, public holding of the fund would ensure that costs are optimally fitted to maximise health impact for the exact same cost we currently pay, rather than for shareholders profits.
If all requirements for such a system already exist and no (major) legal adjustment is required for the system to flourish, then it is fair to wonder why it did not happen earlier. To an extent, it did, with Napster which was later declared illegal in 2001. The major problem which brought Naspter to its end was that while it was not hosting any content itself it was enabling massive copyright infringement by its users who were downloading and listening to music without payment or permission. Spotify solved this issue by intentionally making great efforts to limit that and make their online services function like radio. Radio stations have a \“blanket\” license from something called a \“collecting society\” that permit them to broadcast music without getting a license from each individual music copyright holder. The special legal status with respect to copyright held by radio is the result of decades of negotiation and law-making that created a trade-off between copyright holders like recording companies and radio stations owned by broadcasters.
Note: access means the availability for use if that use is appropriate, it does not mean simple usage. For example, in the case of anti-microbial resistance one may want to limit antibiotics usage in order to preserve efficacy (you could also interpret this as maximizing access over the long-term.)
[return]Rabah Kamal and Cynthia Cox “What Are the Recent and Forecasted Trends in Prescription Drug Spending?” Peterson-Kaiser Health System Tracker; “Health Spending Explorer” Peterson-Kaiser Health System Tracker; “A Look Back at Pharmaceuticals in 2006: Aggressive Advertising Cannot Hide the Absence of Therapeutic Advances”; “New Products and New Indications in 2016: A System That Favours Imitation over the Pursuit of Real Progress.”
[return]Rabah Kamal and Cynthia Cox “What Are the Recent and Forecasted Trends in Prescription Drug Spending?” Peterson-Kaiser Health System Tracker.
[return]Frost, Reich, and others, Access, p. 2; “Delivering on the Global Partnership for Achieving the Millennium Development Goals”, p. 43.
[return]For further information on the different components of access, see Bigdeli, Peters, and Wagner, “Medicines in Health Systems”; Frost, Reich, and others, Access; Peters et al., “Poverty and Access to Health Care in Developing Countries”; Wirtz et al., “Essential Medicines for Universal Health Coverage.”
[return]“GHO | By Category | Antiretroviral Therapy Coverage - Data and Estimates by Country.”
[return]Røttingen et al., “Mapping of Available Health Research and Development Data.”
[return]Wirtz et al., “Essential Medicines for Universal Health Coverage”, p. 456.
[return]Røttingen et al., “Mapping of Available Health Research and Development Data.”
[return]Seddoh et al., “Towards an Investment Case for Neglected Tropical Diseases.”
[return]Smedley, “Is It Fair to Accuse the Pharma Industry of Neglecting Tropical Diseases?”
[return]“Anti-Aging Market (Baby Boomer, Generation X and Generation Y), by Product (Botox, Anti-Wrinkle Products, Anti-Stretch Mark Products, and Others), by Services (Anti-Pigmentation Therapy, Anti-Adult Acne Therapy, Breast Augmentation, Liposuction, Chemical Peel, Hair Restoration Treatment, and Others), by Device (Microdermabrasion, Laser Aesthetics, Anti-Cellulite Treatment and Anti-Aging Radio Frequency Devices) : Global Industry Perspective, Comprehensive Analysis, Size, Share, Growth, Segment, Trends and Forecast, 2015 – 2021.”
[return]Wirtz et al., “Essential Medicines for Universal Health Coverage”, p. 453.
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 87.
[return]Matthijs and Halley, “European-Wide Opposition against the Breast Cancer Gene Patents.”
[return]See Scotchmer, Innovation and Incentives, Chapter 1 on this history.
[return]Technically, from a legal perspective remuneration rights are liability rights whilst patents are more like property rights. However, this distinction
[return]See also Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 6.
[return]Ravvin, “Incentivizing Access and Innovation for Essential Medicines”, p. 115.
[return]“Judging Whether Public Health Interventions Offer Value for Money | Guidance and Guidelines”; Gold, Cost-Effectiveness in Health and Medicine, xx. For other examples, see Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 27.
[return]Prescribing data is more available in developed countries. In other cases one could use data from wholesalers and distributors even if the data was more crude.
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 27.
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 37.
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 40.
[return]Previous submissions of this proposal are Sanders, “Text - H.R.417 - 109th Congress (2005-2006)“; Sanders, “Text - S.2210 - 110th Congress (2007-2008)“; Sanders, “Text - S.1137 - 112th Congress (2011-2012)“; Sanders, “Text - S.1138 - 112th Congress (2011-2012)“; and Sanders, “Text - S.627 - 113th Congress (2013-2014).”
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 37.
[return]“Research and Development to Meet Health Needs in Developing Countries: Strengthening Global Financing and Coordination”, pp. 14-16; “WHO | Meeting the Need for Treatment”; “How a Global R&D Convention Could Fill the Gaps Left by Today’s Medical Innovation System.”
[return]For a more detailed comparison of the remuneration rights system with similar proposals, see Appendix 1.1.
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 2.
[return]Love, “Measures to Enhance Access to Medical Technologies, and New Methods of Stimulating Medical R&D”, p. 704.
[return]“Research and Development to Meet Health Needs in Developing Countries: Strengthening Global Financing and Coordination”, p. 170.
[return]One option in valuing the difference between existing monopoly rights and new remuneration
rights would be an auction process similar to that described in Kremer, “Patent Buyouts.”
[return]As advocated by Aidan Hollis and Thomas Pogge in Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All. For criticism of this approach, see Love and Hubbard, “The Big Idea.”
[return]See Burfisher, Robinson, and Thierfelder, “The Impact of NAFTA on the United States”, Lesher and Miroudot, “Analysis of the Economic Impact of Investment Provisions in Regional Trade Agreements”, pp. 29-32.
[return]See Crafts, “The Growth Effects of EU Membership for the UK: Review of the Evidence.”
[return]“Historical Stock Market Reactions to Geopolitical and Economic Crisis Events.” See also the data on Perlberg, “This Is What Happens To The Stock Market When Terrible Things Happen In The World.”
[return]Frost, Reich, and others, Access, p. 2; “Delivering on the Global Partnership for Achieving the Millennium Development Goals”, p. 43.
[return]MSF, “Untangling the Web of Antiretroviral Price Reductions” 16th Ed., p. 2.
[return]For an overview, see Burt, “Lessons to Be Learnt? - An Overview of Tendering Processes for Medicines across Europe.” Another potential scheme would be reference pricing. See Koskinen et al., “The Impact of Reference Pricing and Extension of Generic Substitution on the Daily Cost of Antipsychotic Medication in Finland” for an example of how this works in practice in Finland.
[return]See “The Swedish Pharmaceutical Reimbursement System” and Kanavos, Seeley, and Vandoros, “Tender Systems for Outpatient Pharmaceuticals in the European Union”, especially p. 35.
[return]Dylst, Vulto, and Simoens, “Tendering for Outpatient Prescription Pharmaceuticals.”
[return]See Boldrin and Levine, Against Intellectual Monopoly, Chapter 9.
[return]Love and Hubbard, “Prizes for Innovation of New Medicines and Vaccines”, p. 171.
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 4.
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 10.
[return]“The Medical Innovation Prize Fund: A New Paradigm for Supporting Sustainable Innovation and Access to New Drugs: De-Linking Markets for Products from Markets for Innovation”, p. 2.
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, pp. 10-11.
[return]Hollis, “An Efficient Reward System for Pharmaceutical Innovation”, p. 6.
[return]“Delivering on the Global Partnership for Achieving the Millennium Development Goals”, p. 35.
[return]Scotchmer, Suzanne. Innovation and Incentives. Cambridge, Mass. ; London: MIT Press, 2004, p. 36.
[return]World health Organisation (WHO), http://www.who.int/hia/about/glos/en/index1.html
[return]See also “Horizon Scan Report” throughout for more detailed information.
[return]“Resolution On Cancer Hailed By WHO Members, Easily Adopted In Committee”, “Geneva Technical Workshop on Proposals for a Cancer Innovation Fund (CIF) – Union for Affordable Cancer Treatment.”
[return]“The Medical Innovation Prize Fund: A New Paradigm for Supporting Sustainable Innovation and Access to New Drugs: De-Linking Markets for Products from Markets for Innovation.”
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All.
[return]“Research and Development to Meet Health Needs in Developing Countries: Strengthening Global Financing and Coordination”, “WHA59.24: Public Health, Innovation, Essential Health Research and Intellectual Property Rights: Towards a Global Strategy and Plan of Action”, “Global Strategy on Public Health, Innovation and Intellectual Property.”
[return]Ravvin, “Incentivizing Access and Innovation for Essential Medicines”, p. 115.
[return]Stiglitz, “Economic Foundations of Intellectual Property Rights”, p. 1724; Love and Hubbard, “The Big Idea”, p. 1553.
[return]An exception is the recent development of direct government grants to small and medium companies, especially in developing economies, for R&D and capacity building. See Paul Cunningham, Abdullah Gök, and Philippe Laredo, “The Impact of Direct Support to R&D and Innovation in Firms.”
[return]Hollis and Pogge, The Health Impact Fund: Making New Medicines Accessible for All, p. 102; Ravvin, “Incentivizing Access and Innovation for Essential Medicines”, pp. 115-116.
[return]Neumann, Using Cost-Effectiveness Analysis to Improve Health Care, p. 8.
[return]Neumann, Using Cost-Effectiveness Analysis to Improve Health Care, p. 8.
[return]Drummond, Methods for the Economic Evaluation of Health Care Programmes, p. 3.
[return]Drummond, Methods for the Economic Evaluation of Health Care Programmes, ch. 6.
[return]